Atoms and Element Worksheet
advertisement
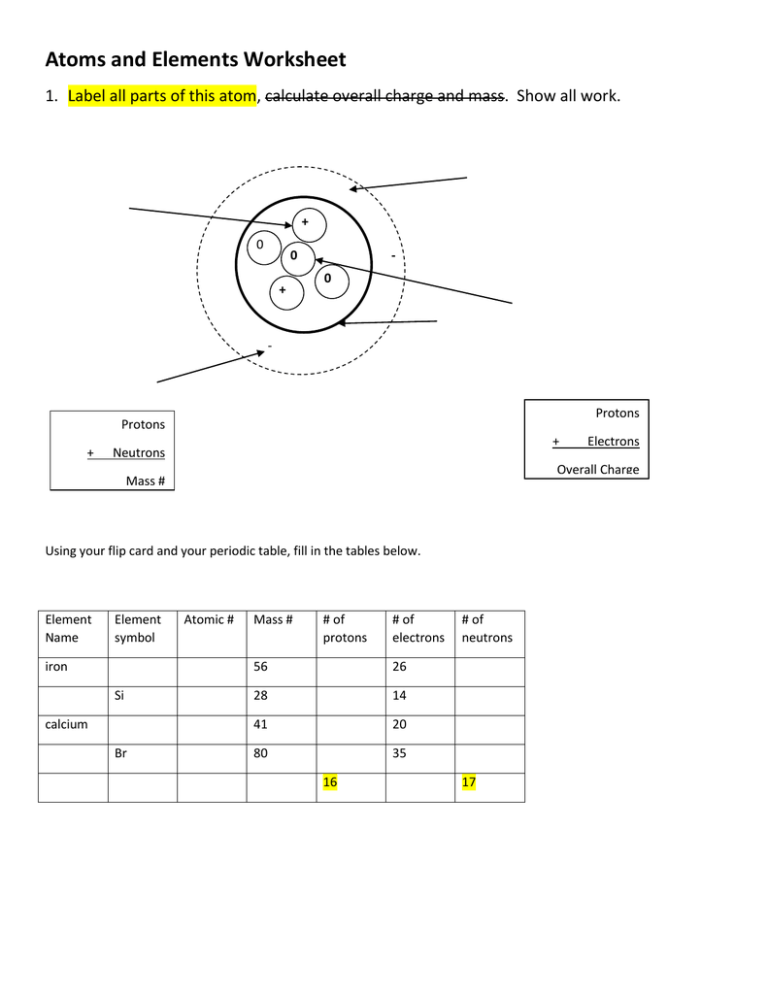
Atoms and Elements Worksheet 1. Label all parts of this atom, calculate overall charge and mass. Show all work. + 0 0 + 0 - Protons Protons + + Neutrons Overall Charge Mass # Using your flip card and your periodic table, fill in the tables below. Element Name Element symbol iron Si calcium Br Electrons Atomic # Mass # # of protons # of electrons 56 26 28 14 41 20 80 35 16 # of neutrons 17 How many protons, electrons, and neutrons do the following atoms have? (fill in the table) Element Name Atomic # Ca Mass # 40 Neutrons Electrons Protons 16 oxygen 17 K 35 39 sulfur 32 sodium 23 Mg 35 C 8 5 35 4 45 Fill in the blanks Matter is anything that has _______________ and _________________. Matter is made up of __________________ which are the building blocks of matter. All atoms must be a type of ______________. Atoms are made up of 3 particles. These 3 particles are _______________________________________________. The __________ and ____________ are found in the nucleus of the atom. The nucleus is in the _______________ of the atom and is very __________________. The charge of the nucleus is _________. The ______________ are found outside the nucleus. These have a _______________charge. The ___________________________ lists all the elements. It is arranged by increasing ___________________________. The atomic number states the number of ____________ an atom has. The mass number of an atom gives the total number of _________________ and _________________. http://thesciences.yolasite.com/chemistry-4th-form.php











