Carbonyl Compounds
advertisement

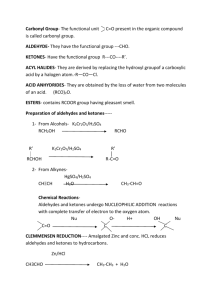
COMPOUNDS CONTAINING A CARBONYL GROUP ALDEHYDES & KETONES Aldehydes and ketones both contain a carbonyl group. The carbon-oxygen bond in the carbonyl group is polar, because oxygen is more electronegative than carbon. The less electronegative carbon atom forms the positive end of the dipole. - + C=O 1. Nucleophilic Addition The electron-deficient carbon atom is susceptible to attack by nucleophiles. In general, substitution occurs at sp3 hybridised carbon atoms, and addition takes place at sp2 hybridised carbon atoms. Since the carbonyl carbon atom is sp2 hybridised, aldehydes and ketones can undergo NUCLEOPHILIC ADDITION. a) Reaction with hydrogen cyanide, HCN Aldehydes and ketones react with hydrogen cyanide to form the corresponding hydroxynitrile. For example, when ethanal reacts with HCN, 2-hydroxypropanenitrile is formed, which contains an asymmetric carbon atom. CH3CHO + HCN CH3C*H(OH)CN The nucleophile is the cyanide ion, CN-, which attacks the + carbon of the carbonyl group. This forms an oxyanion, which subsequently accepts a proton. H3C - H3C C H O :CN - O: H + H3C OH C C CN H CN H 2-hydroxypropanenitrile The carbonyl group is planar, and therefore the attack occurs with equal probability from above or below this plane. Therefore, both enantiomers are formed in equal amounts, giving a racemate. - :CN CN H C* OH CH3 H CH3 C O - :CN OH H C* CN CH3 The reaction of aldehydes and ketones with HCN is useful in synthesis, because it increases the length of the carbon chain by one carbon atom. TOPIC 13.5: COMPOUNDS CONTAINING A CARBONYL GROUP 1 b) Reaction with sodium tetrahydridoborate(III), NaBH4 Aldehydes and ketones are reduced by sodium tetrahydridoborate(III) to the corresponding alcohol. Aldehydes are reduced to primary alcohols and ketones to secondary alcohols. For example, when ethanal reacts with NaBH4, ethanol is formed. CH3CHO + 2[H] CH3CH2OH When propanone reacts with NaBH4, propan-2-ol is formed. CH3COCH3 + 2[H] CH3CH(OH)CH3 Sodium tetrahydridoborate(III) acts as a source of the hydride ion H -. This is a nucleophile and attacks the + carbon of the carbonyl group. This forms an oxyanion, which subsequently accepts a proton from water or an acid. H3C C H - H3C O :H - O: H + H3C C C H OH H H ethanol H NaBH4 is used in aqueous ethanol. A similar reduction takes place with the more powerful reducing agent, lithium tetrahydridoaluminate(III), LiAlH4. This requires scrupulously dry conditions and is usually used in tetrahydrofuran which has been dried with sodium metal. This reduction is specific and will not reduce any alkene double bonds which are present in the same molecule as the aldehyde or ketone. (see CHM3) Catalytic Hydrogenation Reduction of aldehydes and ketones to alcohols can also be brought about by catalytic hydrogenation. The aldehyde or ketone is treated with hydrogen gas in the presence of a finely-divided catalyst , such as Ni or Pt. CH3CHO + H2 CH3CH2OH This reduction is not specific and will reduce any alkene double bonds which are present in a molecule as well as an aldehyde or ketone. 2. Oxidation (see CHM3) Aldehydes are readily oxidised by acidified potassium dichromate(VI) to the corresponding carboxylic acid. The colour change is from orange (Cr 2O72-) to green (Cr3+). CH3CHO + [O] CH3COOH Ketones are not oxidised under these conditions. Since aldehydes are readily oxidised, they behave as reducing agents, whereas ketones do not. This difference allows aldehydes and ketones to be distinguished. TOPIC 13.5: COMPOUNDS CONTAINING A CARBONYL GROUP 2 Tollen’s Reagent Tollen’s reagent is made by adding ammonia solution dropwise to a solution of silver nitrate until the initial brown precipitate of Ag2O just re-dissolves to give a clear colourless solution. This solution contains the ion, [Ag(NH3)2]+. When heated with aldehydes, the complex ion is reduced to silver, which is deposited as a mirror on the walls of the test tube. RCHO + 2[Ag(NH3)2]+ + 3OH- RCOO- + 2Ag + 4NH3 + 2H2O Fehling’s Solution Fehling’s solution is made by adding an alkaline solution of potassium sodium tartrate to copper(II) sulphate solution. This forms a deep blue solution containing a copper(II)-tartrate complex ion. When aliphatic aldehydes are heated with Fehling’s solution, the complex ion is reduced to copper(I) oxide, which appears as a brick-red precipitate. RCHO + 2Cu2+ + 5OH- RCOO- + Cu2O + 3H2O CARBOXYLIC ACIDS Carboxylic acids contain the carboxyl group COOH. C=O O-H A carboxyl group can only occur at the end of a carbon chain. The presence of a carboxyl group is indicated in the name of a compound by the suffix -oic acid. e.g. CH3CH2COOH propanoic acid Preparation Carboxylic acids can be prepared: by the oxidation of aldehydes or primary alcohols RCH2OH + [O] RCHO + H2O RHCO + [O] RCOOH by the hydrolysis of nitriles RCN + 2H2O RCOOH + NH3 Acidity Carboxylic acids are weak acids and dissociate only to a small extent in water. RCOOH RCOO- + H+ In general terms, any structural feature which helps to delocalise the charge on the anion formed from the acid will stabilise it. This has the effect of moving the above equilibrium further to the r.h.s. and increasing the strength of the acid. Carboxylic acids are stronger acids than alcohols, because a mesomeric effect in the carboxylate anion delocalises the charge. .. O- O C O .. O or C O TOPIC 13.5: COMPOUNDS CONTAINING A CARBONYL GROUP 3 C O Carboxylic acids, like most acids, will react with metal carbonates (or hydrogencarbonates) to form carbon dioxide and with reactive metals, such as magnesium, to form hydrogen. 2CH3COOH + Na2CO3 2CH3COONa + H2O + CO2 2CH3COOH + Mg (CH3COO)2Mg + H2 Solubility and Boiling Point Low Mr carboxylic acids are very soluble in water, because the carboxyl group can form hydrogen bonds with water. However, if the length of the carbon chain is increased, the solubility gradually decreases. The presence of hydrogen bonding between carboxylic acid molecules is responsible for their relatively high boiling points. ESTERS Ester contain the functional group COOR, where R represents an alkyl group. They are named as alkyl derivatives of a carboxylic acid. For example: CH3CH2COOH propanoic acid (carboxylic acid) CH3CH2COONa sodium propanoate (salt) CH3CH2COOCH2CH3 ethyl propanoate (ester) Esters are formed by the reaction of a carboxylic acid with an alcohol in the presence of a strong acid catalyst, such as concentrated sulphuric acid. For example: CH3COOH + CH3CH2OH CH3COOCH2CH3 + H2O The reaction is reversible. It is driven to completion by using a small excess of the carboxylic acid and by distilling out the water from the reaction mixture as it is formed. Uses of Esters 1. As solvents Esters do not have any free OH groups and therefore they are unable to form hydrogen bonds, either with other ester molecules or with water. This means that: the boiling points of esters are lower than the boiling points of carboxylic acids of similar Mr esters are almost insoluble in water Ester molecules are, however, polar. They are useful solvents for polar organic compounds and, because of their fairly low boiling points, can easily be removed. This leads to their use in printing inks, lacquers and glues. For example, butyl ethanoate (see practical) is used as a solvent for nitrocellulose. Ethyl ethanoate is used as a solvent in Evostik. 2. As plasticisers The intermolecular forces in thermosoftening plastics are weak, and this allows the plastic to be easily melted and re-shaped. Flexibility in plastics, however, requires the individual polymer chains to be able to move past each other easily. TOPIC 13.5: COMPOUNDS CONTAINING A CARBONYL GROUP 4 This movement occurs easily with, for example, poly(ethene), whose molecules are a long smooth sausage shape. However, the more irregular shape of poly(chloroethene) molecules, caused by the presence of a large chlorine atom on every second carbon atom, makes the molecules lock together. The result is that poly(chloroethene) or p.v.c. is a rigid plastic. Incorporating a plasticiser into this plastic holds the polymer chains further apart, allowing them to move past each other more easily. The plastic, therefore, becomes more flexible. The plastic can contain up to 50% by mass of plasticiser. The esters of benzene-1,4-dicarboxylic acid are widely used as plasticisers for p.v.c. For example: CH2CH3 COOCH2CH(CH2)3CH3 COOCH2CH(CH2)3CH3 CH2CH3 The plasticisers have fairly high boiling points and are not particularly volatile, but over time they may evaporate from the plastic, causing it to become stiffer and more brittle. 3. As Food Flavourings Many aliphatic esters have fruity smells and are used as artificial fruit flavours. Natural flavours are caused by a complex mixture of compounds, and therefore the artificial substitutes are rarely more than an approximation of the real thing. Two examples of esters used as flavourings are: pentyl ethanoate (pear drops) octyl ethanoate (orange) Hydrolysis of Esters 1. Making soap When an ester is hydrolysed, it forms an alcohol and a carboxylic acid. The reaction can be brought about by heating the ester with either dilute aqueous hydrochloric acid (acid-catalysed hydrolysis) or dilute aqueous sodium hydroxide (alkali-catalysed hydrolysis or saponification). The acid-catalysed route is reversible, the alkalicatalysed route is not and therefore tends to be preferred. CH3COOCH2CH3 + NaOH CH3CH2OH + CH3COONa Natural oils and fats are esters of propane-1,2,3-triol (glycerol) and long chain carboxylic acids (fatty acids), which typically have 12 to 20 carbon atoms. Commonly occurring fatty acids are: octadecanoic acid CH3(CH2)16COOH which is saturated and is found in tallow and other animal fats octadec-9-enoic acid CH3(CH2)7CH=CH(CH2)7COOH which is unsaturated and is found in plant oils, such as olive oil When fats and oils are heated with sodium hydroxide solution, they are hydrolysed to form glycerol and a mixture of the sodium salts of the component fatty acids. These salts are soaps. (Saponification means ‘soap making’) TOPIC 13.5: COMPOUNDS CONTAINING A CARBONYL GROUP 5 CH2O.CO.R CHO.CO.R CH2OH + 3NaOH CH2O.CO.R CHOH + 3RCOONa CH2OH Glycerol is a viscous, colourless liquid with a slightly sweet taste. It has three O-H bonds and can, therefore, hydrogen bond easily to other molecules, such as water. Its ability to attract water is put to use in the manufacture of cosmetics and glues. It is used in food and is added to wine to adjust its sweetness and viscosity. 2. Making biodiesel Biodiesel is a fuel made from vegetable oils but can also be made from animal fats. It is made by heating the oils (triesters) with methanol and sodium hydroxide (catalyst). The products are long chain methylesters of fatty acids (biodiesel) and propane1,2,3-triol. CH2O.CO.R CHO.CO.R CH2O.CO.R CH2OH + 3CH3OH CHOH CH2OH TOPIC 13.5: COMPOUNDS CONTAINING A CARBONYL GROUP 6 + 3RCOOCH3