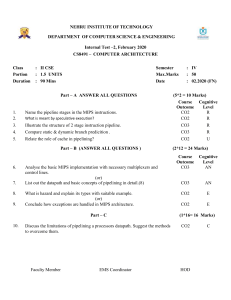
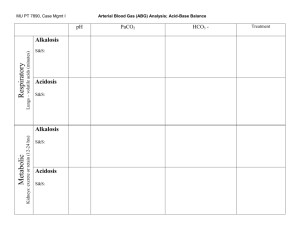
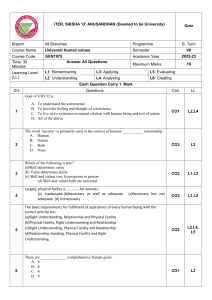
Balancing Equations:
advertisement

Balancing Equations: Given a chemical equation, the species themselves may not be changed. The subscripts in the chemical formula of a compound may not be changed in order to balance the equation. Only the number in front of the species is changed to show a difference in number of atoms, number of moles, number of liters, etc. For example, balance the equation given below: _Al(HCO3)3 (s) _Al2(CO3)3 (s) + _CO2 (g) + _H20 (g) The order of steps below may be changed – this is only my recommendation: 1) do the cations first – so first balance the Al’s There are two Al’s on the right, one on the left, so put a 2 in front of Al(HCO3)3(s) 2) do the C’s next – there are now 6 on the left and 3 in Al2(CO3)3 (s) – need 3 more, so put a 3 in front of CO2(g) 3) have 6H’s on the left side of the equation, so put a 3 in front of H2O(g) 4) do the O’s last 5) count all atoms – it balances, so you are done! Result: 2Al(HCO3)3 (s) Al2(CO3)3 (s) + 3CO2 (g) + 3H20 (g) Practice these: 1) HCl + 2) Fe2O3 3) SO2 4) I2 Zn + + + H2 C O2 Cl2 + Fe ZnCl2 + SO3 ICl3 5) C2H3O2Cl + O2 => CO + H2O + Cl2(g) 6) CO2 + HCl C2H3Cl3 + O2(g) 7) _AgNO3 + _ CaCl2 _ AgCl + _ Ca(NO3)2 CO2