F2 Cl2 Br2 I2 H2 N2 O2
advertisement
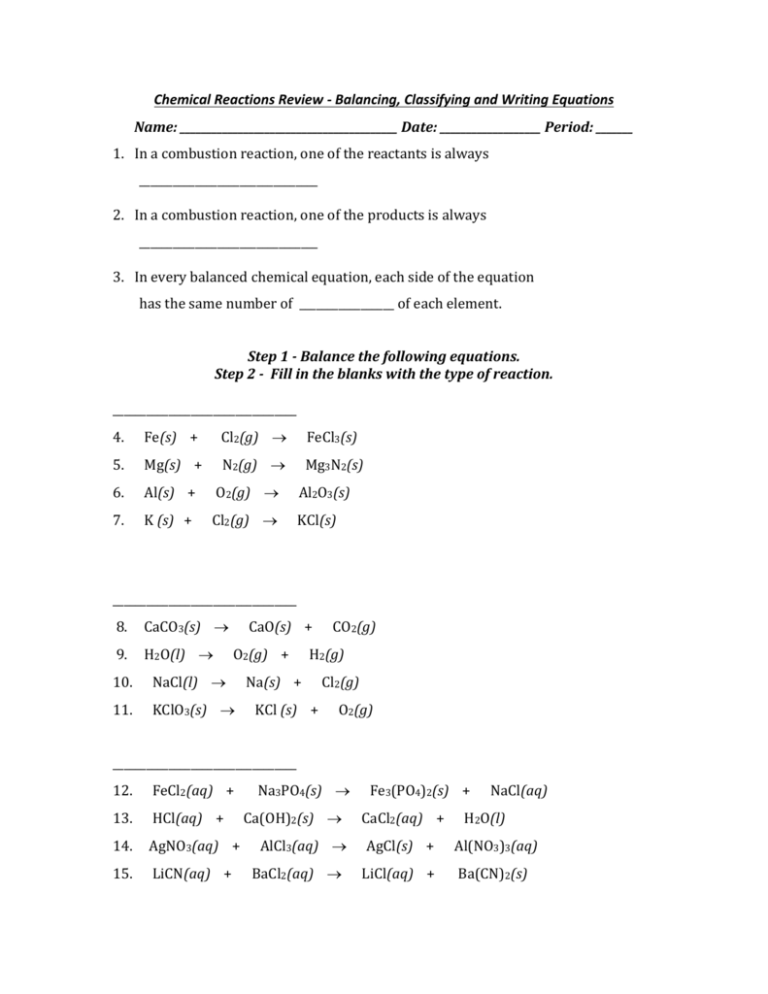
Chemical Reactions Review - Balancing, Classifying and Writing Equations Name: _________________________________________ Date: ___________________ Period: _______ 1. In a combustion reaction, one of the reactants is always ________________________________ 2. In a combustion reaction, one of the products is always ________________________________ 3. In every balanced chemical equation, each side of the equation has the same number of _________________ of each element. Step 1 - Balance the following equations. Step 2 - Fill in the blanks with the type of reaction. _________________________________ 4. Fe(s) + Cl2(g) FeCl3(s) 5. Mg(s) + N2(g) Mg3N2(s) 6. Al(s) + O2(g) Al2O3(s) 7. K (s) + Cl2(g) KCl(s) _________________________________ 8. CaCO3(s) 9. H2O(l) CaO(s) + O2(g) + 10. NaCl(l) 11. KClO3(s) CO2(g) H2(g) Na(s) + KCl (s) + Cl2(g) O2(g) _________________________________ 12. FeCl2(aq) + 13. HCl(aq) + 14. AgNO3(aq) + 15. LiCN(aq) + Na3PO4(s) Ca(OH)2(s) AlCl3(aq) BaCl2(aq) Fe3(PO4)2(s) + CaCl2(aq) + NaCl(aq) H2O(l) AgCl(s) + Al(NO3)3(aq) LiCl(aq) + Ba(CN)2(s) _________________________________ 16. Na(s) + H2O(l) 17. Fe(s) + CuSO4(aq) 18. NaBr(aq) + 19. Sb2S3(s) + NaOH(aq) + Cl2(aq) Fe(s) H2(g) FeSO4(aq) + Cu(s) NaCl(aq) + Br2(l) Sb(s) + FeS(s) _________________________________ 20. CH4(g) + O2(g) CO2(g) + H2O(g) 21. C2H6(g) + O2(g) CO2(g) + H2O(g) 22. C2H2(g) + O2(g) 23. C2H5OH(g) + O2(g) CO2(g) + CO2(g) H2O(g) + H2O(g) For 24 - 28: Write the equation, balance the equation and classify it. F2 Cl2 Br2 I2 H2 N2 O2 24.) The combustion of propane (C3H8). This is a reaction of propane with oxygen that yields carbon dioxide and water. 25.) The reaction of aluminum bromide with magnesium hydroxide to form aluminum hydroxide and magnesium bromide. 26.) Potassium metal and chlorine gas combine to form potassium chloride. 27.) Hydrogen gas and nitrogen monoxide react to form water and nitrogen gas. 28.) The decomposition of hydrogen peroxide (H2O2) to form water and oxygen.











