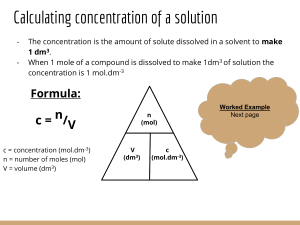
2.0 g of CaCO3 was dissolved in dil. HCl and diluted up to 1000 ml. 30 ml of this solution required 12 ml of EDTA. 30 ml of hard water sample required 10 ml of EDTA. 30 ml of hard water sample on boiling, filtering required 8 ml of EDTA. Calculate temporary & permanent hardness of water sample. A water sample on analysis gave the following data: CaCl2 = 33.3 mg/L; MgSO4 = 48 mg/L; CO2 = 22 mg/L; HCl = 73 mg/L; Mg(HCO3)2 = 73 mg/L, NaCl = 25 mg/L. Calculate the amount of lime (86% pure) and soda (92% pure) required to soften 10000 L of the water sample. (Mol. wt of CaSO4 = 136, MgSO4 =120, CO2 =44, Ca(HCO3)2 =162, NaCl= 58.5). a) An inorganic filter working on adsorption mechanism is cheap and economic. Explain the method. (b) Two methods are used for removing salt from water but one of them uses electric potential and other uses pressure. Differentiate these two methods.