Periodic Trends Project

Periodic Trends Project
The periodic table is an amazing chart. In addition to the elements being listed by increasing atomic number (number of protons in the nucleus), it is also arranged so that the properties of the elements can be easily predicted by looking at the location of an element on the periodic table.
Earlier in the year, we looked at the relationship between electron configuration and location on the periodic table. It was evident that because of the relationship between arrangement of elements and electron configuration the table could be broken up into 4 major sections: the s block, the p block, the d block, and the f block. We also referred to the vertical columns as being families of elements, due the similar properties each element in the column exhibited.
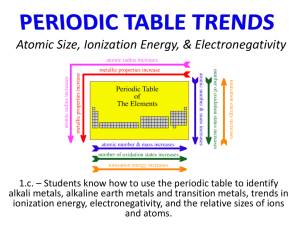
It should not be surprising, when we keep in mind all of the trends we know about so far, that the periodic table contains even more secret information. The properties of the elements are based largely upon the number of protons, neutrons, and electrons contained inside each atom. These properties, which include atomic radius, first ionization energy, and electronegativity, either increase or decrease as you move up, down, left, or right along the rows of the periodic table.
By the end of this project you will be able to use the periodic table to your full advantage, as you will have acquired information about each of the trends. You will also demonstrate understanding of these concepts by using an analogy to create your own periodic table.
Part 1: Research
1. Look up and define these three terms on Google or another search engine: a. Atomic radius: ____________________________________________________________
_________________________________________________________________________ b. First Ionization Energy: ____________________________________________________
_________________________________________________________________________ c. Electronegativity: _________________________________________________________
_________________________________________________________________________
2. Explore the on-line periodic tables found at: atomic radius: http://www.webelements.com/periodicity/atomic_radius/ ionization energy: http://www.webelements.com/periodicity/ionisation_energy_1/ electronegativity: http://www.webelements.com/periodicity/electroneg_allred_rochow/
3. Go to the website for atomic radius. Click on the tab on the picture that says “Shaded
Table.” a. Look at elements Li, Be, and B. As you move from left to right in this row what happens to the atomic radius? b. Look at elements Na, Mg, and Al. As you move from left to right in this row, what happens to the atomic radius? c. Look at elements Li, Na, and K. What happens to atomic radius as you move down this column? d. Look at elements F, Cl, and Br. What happens to atomic radius as you move down this column?
4. Consider the pattern of atomic radius you have observed. Draw points on the ends of the arrows to show increasing atomic radius.
5. Go to the website for ionization energy. Click on the tab on the picture that says “Shaded
Table.” a. Look at elements Li, Be, and B. As you move from left to right in this row what happens to the ionization energy? b. Look at elements Na, Mg, and Al. As you move from left to right in this row, what happens to the ionization energy? c. Look at elements Li, Na, and K. What happens to ionization energy as you move down this column? d. Look at elements F, Cl, and Br. What happens to ionization energy as you move down this column?
6. Consider the pattern of ionization energy you have observed. Draw points on the ends of the arrows to show increasing ionization energy.
7. Go to the website for electronegativity. Click on the tab on the picture that says “Shaded
Table.” a. Look at elements Li, Be, and B. As you move from left to right in this row what happens to the electronegativity? b. Look at elements Na, Mg, and Al. As you move from left to right in this row, what happens to the electronegativity? c. Look at elements Li, Na, and K. What happens to electronegativity as you move down this column? d. Look at elements F, Cl, and Br. What happens to electronegativity as you move down this column?
8. Consider the pattern of electronegativity you have observed. Draw points on the ends of the arrows to show increasing electronegativity.
Part 2: Project
You are to make a poster that shows a group of objects arranged in an analogous fashion to the trends on the periodic table. You may work individually or with ONE partner. The periodic trends must be illustrated correctly, and your topic must be appropriate for school.
How to get started:
First, select a group of 25* related objects (ex: pokemon, sports players, types of cars).
Then, arrange those items into a pattern that increases or decreases in the same general pattern as two of the three periodic trends you learned about.
Create a poster, being sure to include these criteria:
1.
Contains 25* objects.
2.
Objects are arranged in a pattern that mimics the direction of increase or decrease of at least two periodic trends.
3.
Arrows show the direction of increase, and are labeled as to which properties of the objects are increasing.
4.
A title is on the poster that gives and indication of which trend and what type of objects you are using.
5.
A paragraph is included that discusses, clearly and completely, the analogy between the periodic trend and the properties that increase among your objects.
(ex: gas mileage is like first ionization energy because ionization energy is the amount of energy needed to become an ion, while gas mileage is the amount of gas needed to go a mile.)
6.
Poster is neat, colorful, and shows a great deal of effort.
*