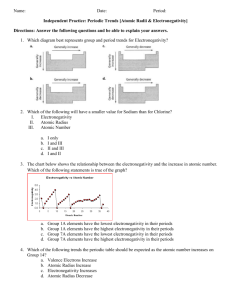
Atomic Radius and Electronegativity Questions
advertisement
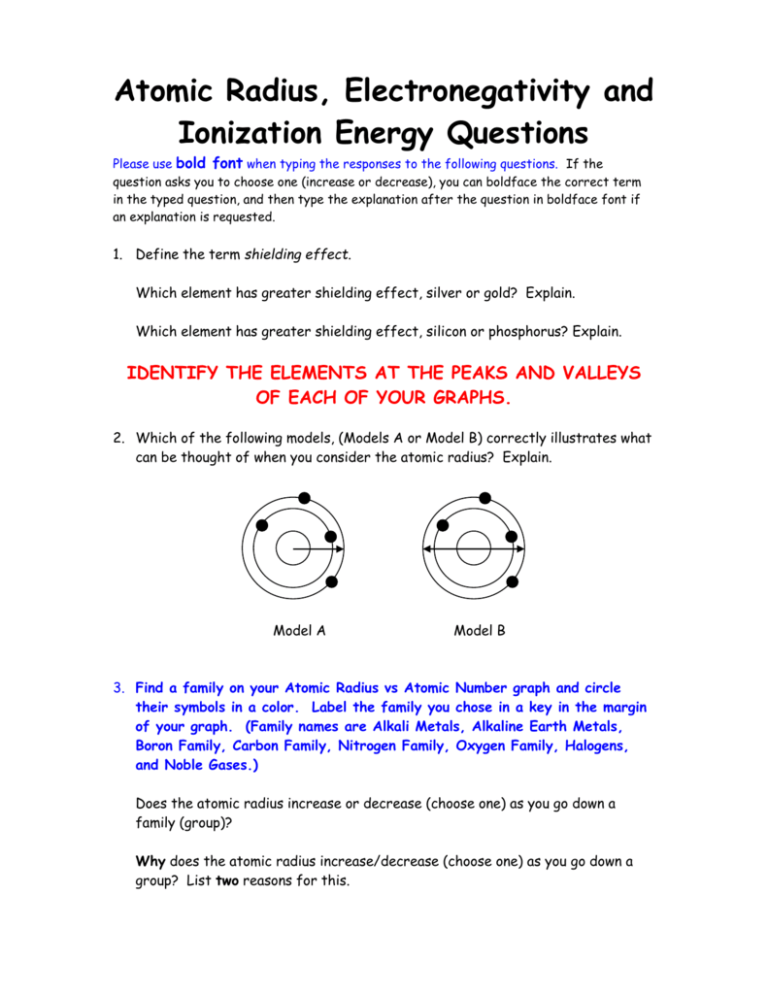
Atomic Radius, Electronegativity and Ionization Energy Questions Please use bold font when typing the responses to the following questions. If the question asks you to choose one (increase or decrease), you can boldface the correct term in the typed question, and then type the explanation after the question in boldface font if an explanation is requested. 1. Define the term shielding effect. Which element has greater shielding effect, silver or gold? Explain. Which element has greater shielding effect, silicon or phosphorus? Explain. IDENTIFY THE ELEMENTS AT THE PEAKS AND VALLEYS OF EACH OF YOUR GRAPHS. 2. Which of the following models, (Models A or Model B) correctly illustrates what can be thought of when you consider the atomic radius? Explain. Model A Model B 3. Find a family on your Atomic Radius vs Atomic Number graph and circle their symbols in a color. Label the family you chose in a key in the margin of your graph. (Family names are Alkali Metals, Alkaline Earth Metals, Boron Family, Carbon Family, Nitrogen Family, Oxygen Family, Halogens, and Noble Gases.) Does the atomic radius increase or decrease (choose one) as you go down a family (group)? Why does the atomic radius increase/decrease (choose one) as you go down a group? List two reasons for this. 4. Find a period on your Atomic Radius vs Atomic Number graph and circle their symbols in second (different) color. Label the period you chose in the key in the margin of your graph. (Periods are numbered 1st, 2nd, 3rd, etc.) Does the atomic radius increase or decrease (choose one) as you go across a period? Why does the atomic radius increase/decrease (choose one) as you go across a period? 5. What is meant by the term electronegativity? 6. Find a family on your Electronegativity vs Atomic Number graph, and circle their symbols in a color. Label the family you chose in a key in the margin of your graph. Does the electronegavitiy increase or decrease (choose one) as you go down a family (group)? Why does the electronegativity increase/decrease (choose one) as you go down a group? List two reasons for this. 7. Find a period on your Electronegativity vs Atomic Number graph and circle their symbols in a second (different) color. Label the period you chose in the key in the margin of your graph. Does the electronegativity increase or decrease (choose one) as you go across a period? Why does the electronegativity increase/decrease (choose one) as you go across a period? 8. What is meant by the term ionization energy? 9. Find a family on your Ionization Energy vs Atomic Number graph and circle their symbols in a color. Label the family you chose in a key in the margin of your graph. Does the ionization energy increase or decrease (choose one) as you go down a family (group)? Why does the ionization energy increase/decrease (choose one) as you go down a group? List two reasons for this. 10. Find a period on your Ionization Energy vs Atomic Number graph, and circle their symbols in a second (different) color. Label the period you chose in the key in the margin of your graph. Does the ionization energy increase or decrease as you go across a period? Why does the ionization energy increase/decrease (choose one) as you go across a period?