Atomic Size, Ionization Energy, & Electronegativity
advertisement

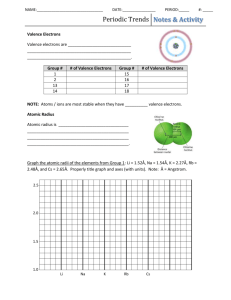
Atomic Size, Ionization Energy, & Electronegativity 1.c. – Students know how to use the periodic table to identify alkali metals, alkaline earth metals and transition metals, trends in ionization energy, electronegativity, and the relative sizes of ions and atoms. • Mendeleev understood the ‘Periodic Law’ which states: • Atoms with similar properties appear in groups or families (vertical columns) on the periodic table. • They are similar because they all have the same number of valence (outer shell) electrons, which governs their chemical behavior. • There are several other important atomic characteristics that show predictable trends that you should know. 1. Atomic size 2. Ionization Energy 3. Electronegativity • Atoms at the bottom of the periodic table are BIGGER than atoms at the top! 1 2 3 4 5 6 E7 E5 7 E3 E2 E1 nucleus E4 E6 • As we go down a group each atom has another energy level…So the atoms get bigger. H Li Na K Rb • Atoms on the left side of the periodic table are bigger than atoms on the right side of the periodic table. • As we move across a period….protons are added….this increases the nuclear charge (+)! • As protons are added….electrons are also added….so the electrostatic attraction increases (positive & negative attraction)! • This attraction pulls the outermost electrons in Nato the nucleus….making Mg Al Si thePatomSsmaller! Cl Ar closer _ _ ++ • Atomic size tends to increase from right to left and from top to bottom. • Ionization Energy - amount of energy needed to remove an electron from an atom. • It is harder to remove an electron from smaller atoms because the nucleus is closer and better able to hold on to them. • It is easier to take electrons from larger atoms because the electrons are further from the nucleus. • It is easier to take electrons from metals since they want to LOSE electrons! LOW IONIZATION ENERGY Medium-High IONIZATION ENERGY Very High IONIZATION ENERGY • Ionization Energy tends to increase from left to right and from bottom to top. • Electronegativity - ability of an atom to attract an electron. • It is easier for small atoms to attract an electron because the nucleus is closer and is better able to hold on to them. • It is easier for nonmetals to gain electrons. • Electronegativity tends to increase from left to right and from bottom to top. Metals Metalloids (Semimetals) Nonmetals Left Side Alkali Alkaline Earth Transition Elements dividing the metals and nonmetals (stair-step line) Right Side Halogens Noble Gases Give up (Lose) eCations Give up (Lose) eOr Accept (Gain) e- Accept (Gain) e(Noble gases do not gain e-) Anions Big Atomic Size Small Atomic Size Low Ionization High Ionization Low Electronegativity High Electronegativity (Excluding Noble Gases) Cornell Questions • As you move across a Period, how does the atomic number change? • As you move across a Period, how does the “pull” of the nucleus on the electrons change? • As you move across a Period, how does the atomic size, ionization energy, and electronegativity change? • As you move down a Group, how does the atomic size, ionization energy, and electronegativity change? Create a Periodic Table Trends Periodic Table 1. Number the rows and groups 2. Draw increasing arrows representing atomic size (must be in color) 3. Draw increasing arrows representing ionization energy (must be in a different color) 4. Draw increasing arrows representing electronegativity (must be in a different color)