Trends in the Periodic Table
advertisement

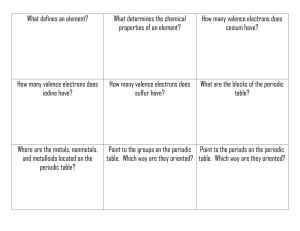
Title: Trends in the Periodic Table Introduction/Purpose: Pre Lab; 1. Look up the history of the periodic table and the contributions made by Dmitri Mendeleev and Henry Moseley. 2. Look up the following terms: Periodicity, group, period, electronegativity, atomic radius, ionization energy, valence electrons Materials: Graph paper, ruler, CRT Methods/Procedure: Explain how to make a graph (scale, title, units, etc) 1. Graph atomic numbers 1-18 (x axis) verse ionization energy, atomic radius, electronegativity. (y axis)Total of 3 graphs. Make it nice nice. Use an appropriate scale. Observations/Data/Diagrams: Explain what observations you can make from the graphs. Identify relationships between the variables, going down a group, across a period, verse each other, etc. Discussion/Analysis: NA Conclusion: Answer the following questions: 1. 2. 3. 4. 5. 6. What is the relationship between ionization energy as you go; a. across a period, b. down a group? Why? Look it up. What is the relationship between atomic radius as you go; a. across a period, b. down a group? Why? Look it up. What is the relationship between electronegativity as you go; a. across a period, b. down a group? Why? Look it up. What is the relationship between ionization energy and atomic radius? Direct/indirect What is the relationship between ionization energy and electronegativity? Direct/indirect What is the relationship between atomic radius and electronegativity? Direct/indirect Figures and Graphs: Include 3 graphs