Chemical Bonding: Dot-Cross Diagrams & Properties
advertisement

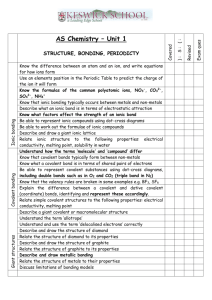
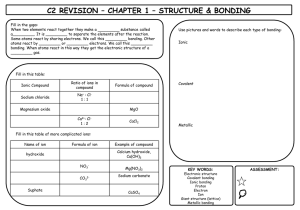
The New Progress Tracker For each section of the module you will be given a progress tracker booklet. This is designed to enable you to sign of when a section of the specification is complete or has been reviewed and to allow communication between you and your teachers about the best way to help you to progress. F332 – Chemistry of Natural Resources • This module is made up for three main topics ▫ Elements from the Sea ▫ The Atmosphere ▫ Polymer Revolution • This is the biggest module this year, worth 50% of your AS level, therefore 25% of the full A-level Dot-Cross Diagrams And Introducing Dative Bonding Objectives Be able to... • Draw simple dot-cross diagrams for ionic and covalent compounds • Describe and draw dative covalent bonds • Link the types of bonding to physical properties of chemicals Specification... Where this comes from... Types of bonding There are three types of bond that can occur between atoms: an ionic bond occurs a covalent bond between a metal and occurs between two non-metal atom (e.g. non-metal atoms (e.g. NaCl) I2, CH4) a metallic bond occurs between atoms in a metal (e.g. Cu) Representing ionic bonding Covalent bonding Task • Draw dot-cross diagrams for: 1. 2. 3. 4. 5. Lithium fluoride Calcium chloride Water Ethene Phosphorus pentachloride (beware this does not obey the octet rule) Lithium Fluoride Calcium chloride Water Ethene Phosphorus Pentachloride Co-ordinate bonding Examples of co-ordinate bonds Task • Explain what a dative covalent bond is and how it is formed. Give examples of molecular ions containing dative covalent bonds. • Draw dot-cross diagrams for: ▫ Hydroxonium ion ▫ Ammonium chloride Hydroxonium Ion Ammonium Chloride Bonding and Properties • How do the properties of chemicals differ within different types of bonding? • Consider: ▫ ▫ ▫ ▫ ▫ Melting and Boiling Points Solubility Thermal and electrical conductivity Hardness Any others you can think of... • Discuss. Bonding and Properties Property Melting and Boiling Points Solubility Thermal and electrical conductivity Hardness Other points Ionic Simple Molecular Giant Covalent