Chemical Bonding
advertisement

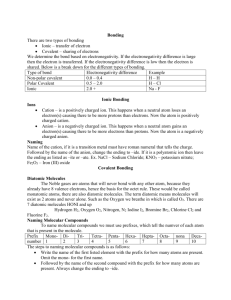
Chemical Bonding In compounds, atoms are held together by forces known as chemical bond. Electrons play a key role in chemical bonding. An electron in an atom can be characterized by quantum numbers. 1. Principal quantum number (n) n = 1, 2, 3, …. K L M 2. Orbital quantum number (l) l = 0, 1, 2, 3, …. n-1 s p d f 3. Magnetic quantum number (ml) -l, o, +l 4. Electron spin: + 1/2 or – 1/2 Shorthand notation to designate electron configuration: spdf notation: 8O 13Al 1s2 2s2 2p4 1s2 2s2 2p6 3s2 3p1 O Al 1s 2s 2p 1s 2s 2p inner-shell electrons Kernel of the atom Al Lewis symbol orbital diagram: 3s 3p outer-shell electrons Valence electrons Lewis theory: 1. Electrons of the outermost (valence) electron shell play a fundamental role in chemical bonding. 2. Chemical bonding may result from the transfer of one or more electrons from one atom to another. This type of bond known as ionic. 3. Chemical bonding may result from a mutual sharing of electrons between atoms. This bond type called covalent. 4. Transfer or sharing of electrons results in noble gas configuration, that is, often an octet. First ionization energy Ionic bond: Electron affinity Na Cl Na+ Na + Cl Na+ + Cl Cl- 1. Ionic bond results from the transfer of electrons between a metal and a nonmetal. 2. The nonmetal atom gains a sufficient number of electrons to produce an anion with noble gas electron configuration. 3. In solid state an enormous number of in cluster together into a crystalline solid. 4. Formula unit: the smallest collection of ions that is electrically neutral. Covalent bonding involves sharing of electrons H + Cl H Cl Single covalent bonding: H + H Cl + Cl H H Cl Cl or H H Nonpolar: DE.N. < 0.6 Multiple covalent bonding: N N O O N N O O NH3 + H+ NH4+ H H H H N + H+ Polar: 0.6 < DE.N < 2.1 Ionic: DE.N > 2.1 H + N H H Coordinate covalent bond