Covalent Bonding in Methane, Ethane, Ethene: Chemistry Notes
advertisement
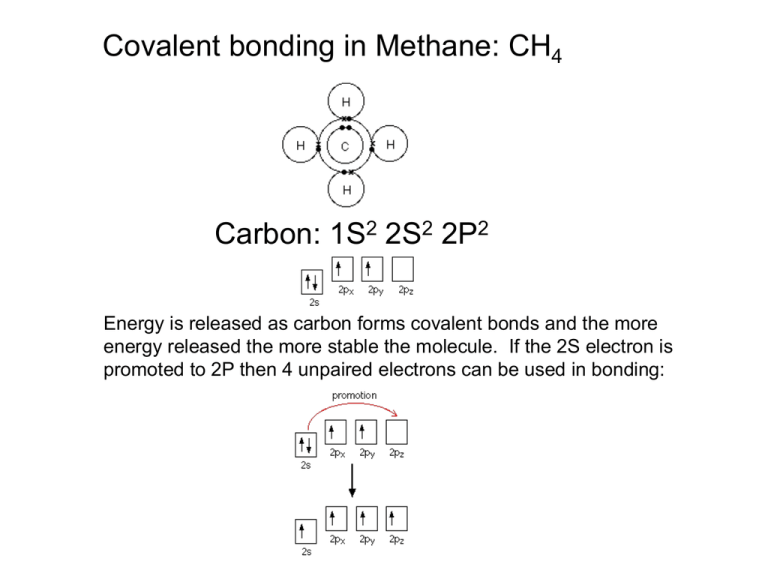
Covalent bonding in Methane: CH4 Carbon: 1S2 2S2 2P2 Energy is released as carbon forms covalent bonds and the more energy released the more stable the molecule. If the 2S electron is promoted to 2P then 4 unpaired electrons can be used in bonding: In methane, all four C-H bonds are identical, so the electrons in the S and P orbitals rearrange themselves in a process called hybridization hybridization The SP3 orbitals are tetrahedral in shape to avoid electron repulsion between orbitals and this defines the shape of CH4 where it can bond with 4 hydrogen atoms. Bond angle 109o H H C 109 H H In ethane, C2H6 there are 4 SP3 orbitals for each carbon atom available for bonding; Three between C and H and the remaining one between C and C forming a sigma (s) bond: Ethene, CH2CH2 has a different bonding structure: Three of the 2S and 2P electrons are hydridized this time, to leave a 2P orbital. Then three SP2 hybrids form: Before C=C bonding Bonds forming (molecular orbitals)