Section 3 - Bonding and Structure powerpoint
advertisement

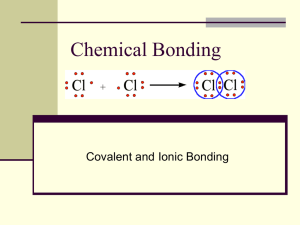
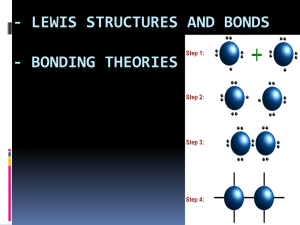
Covalent bonding Learning Objectives Candidates should be able to: describe, including the use of ‘dot-and-cross’ diagrams, covalent bonding, as in hydrogen; oxygen; chlorine; hydrogen chloride; carbon dioxide; methane; ethene. describe covalent bonding in terms of orbital overlap. Starter Activity Forming a bond Shared pair of electrons Electron density map Electron density maps for a hydrogen molecule Representing a covalent bond This single σ covalent bond can be simply represented as: or H – H Overlap of atomic orbitals Overlap of two p-orbitals – e.g. F2 Overlap of one s and one p orbital – e.g. HF Dot-cross diagrams Oxygen Carbon dioxide Chlorine Methane Hydrogen chloride Ethene Breaking the octet rule Unexpected structures !! Bonding in CH4 – promotion of an electron C 1s2 2s2 2p2 Bonding in CH4 – hybridisation Hybridisation in PCl5 Co-ordinate bonding Learning Objectives Candidates should be able to describe, including the use of ‘dot-and-cross diagrams, co-ordinate (dative covalent) bonding, as in the formation of the ammonium ion and in the Al2Cl6 molecule. A co-ordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom. Starter Activity Dot-cross diagrams H2S SiCl4 CH3OH SF6 CO Reaction between NH3(g) and HCl(g) Reaction between NH3(g) and HCl(g) Reaction between H2O and HCl Electron-deficient BF3 Aluminium chloride vapour Aluminium chloride vapour Electronegativity Learning Objectives Candidates should be able to explain the origin of polar bonds, with reference to electronegativity differences between atoms. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity values Starter Activity Why are bonds like bears? …’cos some of them are POLAR! Electronegativity differences What happens if two atoms electronegativity bond together? What happens if B electronegative than A? is of slightly equal more Representing polar bonds Representing polar bonds Molecule Electronegativity difference Dipole/debye HCl 0.9 1.03 HBr 0.7 0.78 HI 0.4 0.38 Large electronegativity difference What happens if B is a lot more electronegative than A? An ionic bond is formed!!! Type of bond As a rough guide: Type of bond Non-polar Covalent Polar covalent Ionic Electronegativity difference 0.5 Between 0.5 and 1.7 1.7 Ionic with increasing covalent character Polarisation of Anions Truly ionic Positive cation Negative anion Ionic with some covalent character Polarisation of Anions Property Charge Radius Cation is the most powerful polarising agent when.... Anode is most easily polarised when... High High Small Large Do polar bonds make polar molecules? CCl4 molecule is tetrahedral - the partial negative charges on the Cl atoms are distributed pretty symmetrically around the molecule. The partial positive charge on the C is buried in the center of the molecule. The most electronegative element is fluorine. If you remember that fact, everything becomes easy, because electronegativity must always increase towards fluorine in the Periodic Table. Trends in electronegativity Ionic bonding Learning Objectives Candidates should be able to describe ionic (electrovalent) bonding, as in sodium chloride and magnesium oxide, including the use of ‘dot-and-cross’ diagrams. “The name’s Bond, Ionic Bond – taken, not shared!!! Starter Activity Approaching atoms Ionic bonding Ionic bonding MgO K2O CaCl2 Al2O3 Why CaCl2 and not CaCl or CaCl3? Why CaCl2 and not CaCl or CaCl3? Polarisation of Anions Truly ionic Positive cation Negative anion Ionic with some covalent character Polarisation of Anions Property Charge Radius Cation is the most powerful polarising agent when.... Anode is most easily polarised when... High High Small Large Shapes of molecules Learning Objectives Candidates should be able to explain the shapes of, and bond angles in, molecules by using the model of electron-pair repulsion. Starter Activity Starter Activity 180o Linear BeCl2 Balloon molecules 2 Balloons give a linear geometry 3 Balloons give a trigonal planar geometry 4 Balloons give a tetrahedral geometry 5 Balloons give a trigonal bipyramidal geometry 6 Balloons give an octahedral geometry Shapes of molecules Electrons in Electrons No. of pairs No. of Diagram of outer shell of added from of electrons bonding pairs molecule central atom other atoms (including bond (and any angles) charge on an ion) Description of shape BF3 3 3 3 3 Trigonal planar CCl4 4 4 4 4 Tetrahedral NH3 5 3 4 3 Trigonal pyramidal H2O 6 2 4 2 Bent (or Vshaped) Shapes of molecules SF6 6 6 6 6 Octahedral CO2 4 4 2 2 Linear C2H6 4 4 4 4 Tetrahedral C2H4 4 4 3 3 Trigonal planar ClF4- 7 5 6 4 Square planar Metallic bonding Lesson Objectives Candidates should be able to describe metallic bonding in terms of a lattice of positive ions surrounded by mobile electrons. Starter Activity Metallic Bonding This is sometimes described as "an array of positive ions in a sea of electrons". Close packed structures? Dense metals Group 1 metals Malleability Metal grains Intermolecular forces Lesson Objectives Candidates should be able to describe intermolecular forces (van der Waals’ forces), based on permanent and induced dipoles, as in CHCl3(l), Br2(l) and the liquid noble gases. Starter Activity Particles in solids, liquids and gases In a liquid or a solid there must be forces between the molecules causing them to be attracted to one another, otherwise they would move apart from each other and become a gas. Intermolecular forces Intermolecular attractions are attractions between one molecule and a neighbouring molecule. How do intermolecular (or van der Waals) forces arise? Temporary or instantaneous dipoles The lozenge-shaped diagram represents a small symmetrical molecule - H2, perhaps, or Br2. The even shading shows that on average there is no electrical distortion (i.e. the molecule is non-polar). Temporary or instantaneous dipoles Electrons are mobile. The constant "sloshing around" of the electrons in the molecule causes rapidly fluctuating dipoles even in the most symmetrical molecule. It even happens in monatomic molecules - molecules of noble gases, like helium, which consist of a single atom. Temporary dipole - induced dipole interaction How temporary dipoles give rise to intermolecular attractions van der Waals’ forces This diagram shows how a whole lattice of molecules could be held together in a solid using van der Waals’ forces. An instant later, of course, you would have to draw a quite different arrangement of the distribution of the electrons as they shifted around - but always in synchronisation. van der Waals lattice How molecular size affects the strength of the dispersion forces The boiling points of the noble gases are: helium -269°C neon argon krypton xenon radon -246°C -186°C -152°C -108°C -62°C How molecular size affects the strength of the dispersion forces There is a gradual increase in the very low boiling temperatures of the noble gases with increasing atomic size. As the size of the atoms increases the number of electrons increases and the magnitude of the van der Waals forces increases. How molecular shape affects the strength of the temporary dipole interactions Butane has a higher boiling point because the intermolecular forces are greater. The molecules are longer and can lie closer together than the shorter, fatter 2methylpropane molecules. Permanent dipoles Permanent dipoles Hydrogen bonding Lesson Objectives Candidates should be able to describe hydrogen bonding, using ammonia and water as simple examples of molecules containing N-H and O-H groups. Starter Activity Boiling points of the Group 4 hydrides The increase in boiling point happens because the molecules are getting larger with more electrons, and so van der Waals forces become greater. Boiling points of the hydrides in Groups 5, 6 and 7. The origin of hydrogen bonding The origin of hydrogen bonding Hydrogen bonding is a particularly strong intermolecular force that involves three features: a large dipole between an H atom and the highly electronegative atoms N, O or F; the small H atom which can get very close to other atoms; a lone pair of electrons on another N, O or F, with which the positively charge H atom can line up. Drawing hydrogen bonds 1 mark for showing H-bond 1 mark for showing lone pair 1 mark for indicating bond polarity Hydrogen bonding in water Hydrogen bonding accounts for many of the other unusual properties of water including: its high specific heat capacity its very high surface tension its high viscosity and the low density of ice compared to water Which type of intermolecular force? A summary Bonding, structure and properties Lesson Objectives Candidates should be able to: describe, interpret and/or predict the effect of different types of bonding on the physical properties of substances. describe, in simple terms, the lattice structure of a crystalline solid which is ionic, simple molecular, giant molecular, hydrogen-bonded and metallic. suggest from quoted physical data the type of structure and bonding present in a substance. Starter Activity Bonding, structure and properties The properties of substances are decided by their bonding and structure. Bonding means the way the particles are held together: ionic, covalent, metallic or weak intermolecular bonds. Structure means the way the particles are arranged relative to one another. You have already met the major types of structure at IGCSE. Bonding, structure and properties Bond energies Bond Average bond Bond length/nm enthalpy/kJmol-1 C – C +347 0.154 C = C +612 0.134 C ≡ C +838 0.120 C – H +413 0.108 O – H +464 0.096 C – O +358 0.143 C = O +805 0.116 GIANT LATTICE COVALENT MOLECULAR Ionic Covalent network Metallic What substances have Compounds of metals Some elements in Metals this type of with non-metals. Group 4 and some of structure? their compounds. Examples What type of particle does it contain? How are the particles bonded together? NaCl ions SiO2 atoms Strong ionic bonds; attraction between oppositely charged ions Strong covalent bonds; attraction of atoms’ nuclei for shared electrons high very high generally high low hard but brittle hard but malleable conduct when (s) or (l) soft conduct when (l) or (aq) very hard (if 3D) do not normally conduct often soluble insoluble insoluble (but insoluble insoluble insoluble What are the typical properties? M. pt and b.pt. Hardness Electrical conductivity Solubility in water Solubility in non-polar solvents (e.g. hexane) Cu positive ions and delocalised electrons Simple molecular Macromolecular Some non-metal Polymers elements and usually some non-metal/nonmetal compounds. H2 O molecules Poly(ethene) molecules Strong metallic Weak intermolecular bonds between bonds; attraction molecules; strong covalent bonds of atoms’ nuclei between atoms within each molecule. for delocalised electrons some react) moderate (often decompose on heating) variable do not conduct do not normally conduct sometimes usually insoluble (but some H-bond) soluble sometimes usually soluble soluble Structure table The modern use of materials Lesson Objectives Candidates should be able to: Explain the strength, high melting point and insulating properties of ceramics in terms of their giant molecular structure. Relate the uses of ceramics to their properties. Describe and interpret the uses of the metals aluminium and copper (and their alloys) in terms of their physical properties. Understand that materials are a finite resource and the importance of recycling processes. Starter Activity Five most common metals Aluminium Brass Zinc Copper Steel Uses of aluminium Low density, corrosion resistance and strength make it ideal for construction of aircraft, lightweight vehicles, and ladders. Malleability, low density, corrosion resistance and good thermal conduction make it a good material for food packaging. Good electrical conduction, corrosion resistance and low density leads to its use for overhead power cables hung from pylons (low density gives it an advantage over copper). Uses of copper Copper is an excellent conductor of electricity and heat. Copper is soft and malleable. Copper is very unreactive and therefore corrosion resistant. Alloys of copper Copper Alloy Other metal it contains Brass Zinc Bronze Tin Main properties Uses Fairly soft and malleable Screws and hinges Strong Propellors and bearings Ceramics A mineral is a naturally occurring solid formed through geological processes that has a characteristic chemical composition, a highly ordered atomic structure, and specific physical properties. Ceramic: Any of various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing a nonmetallic mineral. Ceramics furnace: an enclosed chamber in which heat is produced to heat buildings, destroy refuse, smelt or refine ores, etc. heat shields glass and crockery brake pads furnace linings electrical insulators Recycling Raw materials extracted (removed by chemical means) from the Earth cannot last forever. Although some materials are more (present in great quantity) abundant than others, they are all finite (have a limit) resources. Increasing demand for raw materials (items used to produce something else), coupled with ever growing problems of waste disposal, have led to considerable interest in recycling (processing for reuse) waste. Recycling has a number of possible advantages (beneficial factors): It leads to reduced demand for new raw materials; It leads to a reduction in environmental damage (harm to the surroundings); It reduces the demand for landfill sites (a place for burying waste) to dump waste; It reduces the cost of waste disposal; It may reduce energy costs. The kinetic-molecular model of liquids Lesson Objectives Candidates should be able to describe using a kineticmolecular model, the liquid state; melting; vaporisation and vapour pressure. Starter Activity The Kinetic-Molecular Model of Liquids Solid Arrangement of very orderly particles Liquid Gas almost complete disorder high Movement of particles vibrate about fixed positions Proximity of particles Compressibility of substance Conduction of heat close (~10-10m) short-range order, longer range disorder some movement from place to place close (~10-10m) very low very low poor except metals and graphite metals very good; very poor others poor continuous, rapid, random movement far apart (~10-8m) The Kinetic-Molecular Model of Liquids Liquids do not have a fixed __________ because the particles can move about. However, they remain very __________ together. This shows that the inter-particle forces have not been __________ broken. If sufficient __________ is supplied, the particles overcome the interparticle forces almost completely and __________ from the liquid. This is called __________ or boiling. The energy required to boil a liquid is always __________ than that required to melt the same substance and is a better __________ of the strength of inter-particle forces. Vapour pressure Vapour pressure is the pressure of a vapour over a liquid at equilibrium. Vapour pressure Even at low temperature there are particles with high energy. Vapour pressure At equilibrium, the rate at which molecules leave the liquid equals the rate at which molecules join the liquid. Measuring vapour pressure More on ideal gases Lesson Objectives Candidates should be able to explain qualitatively in terms of intermolecular forces and molecular size the limitations of ideality at very high pressures and very low temperatures. Starter Activity More on ideal gases More on ideal gases Y is hydrogen. It is closest to ideal under all conditions. Hydrogen has the weakest intermolecular forces and is the smallest molecule. Z is ammonia. It is the least ideal at lower pressures. Ammonia molecules can hydrogen bond. X is nitrogen. Deviates greatly from ideality at high pressures where its larger molecular volume becomes important.