Unit 4 Review PowerPoint - Dr. Vernon-
advertisement

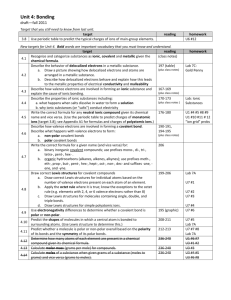
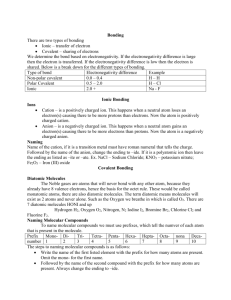
How many valence electrons does magnesium have? 2 How many valence electrons does nitrogen have? 5 What is the oxidation number of Oxygen? -2 Which is the only noble gas without 8 valence electrons? He How many electrons would Fluorine like to lose or gain to become fluoride? Gain 1 What is the charge of a Fe (II) ion? +2 What is the name of K2O? Potassium oxide What is the name of SiO2? Silicon dioxide What is the name of Cu2O? Copper (I) oxide What is the formula for sodium sulfate? Na2SO4 What is the formula (with charge) for a phosphate ion? PO4 3- In Beryllium Chloride (BeCl2), which element is losing electrons? How many? Be loses 2 What is happening with the electrons in a single covalent bond? 2 electrons are shared between atoms What is the EN difference in a Carbon-Oxygen bond? Is that polar or non? 0.9, polar Why is CO2 a non-polar MOLECULE? Symmetrical; dipoles cancel What symbol do we use for a dipole? + What are the electrons doing in a POLAR covalent bond? Being shared UNEQUALLY Is TiO ionic or covalent? Ionic Is NO ionic or covalent? Covalent Is NH4OH ionic or covalent? Ionic What is the name of NH4OH? Ammonium hydroxide What is the formula for nickel (II) carbonate? NiCO3 How many domains does the carbon in CH4 have? 4 What is the name of the shape CH4 has? Tetrahedral How many domains does the carbon in NH3 have? 4 What is the name of the shape NH3 has? Trigonal pyramidal Which element only needs 6 electrons (instead of an octet)? Boron Why is a water molecule bent instead of linear? Lone pairs Which is the strongest intermolecular force? Molecule-ion interaction Which intermolecular force is responsible for water’s high boiling point? Hydrogen bonding Hydrogen bonding is an extra strong example of what other intermolecular force? Dipole-dipole interaction Which intermolecular force is present with nonpolar compounds? Dispersion forces One of which 3 elements needs to be attached to hydrogen for hydrogen bonding to occur? N, O, or F