- Lewis structures and bonds
advertisement

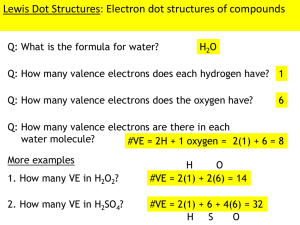
- LEWIS STRUCTURES AND BONDS - BONDING THEORIES Lewis Structures and Bonding Use NASB to draw dot diagrams. N – electrons needed to fill valence (8 or 2) A- electrons available in atom S – electrons shared = N – A B – formed bonds (S divided by 2) In Cl2, the total number of unshared pairs of electrons is 6. Cl + Cl Cl―Cl or Cl:Cl The diatomic molecule N2 contains a triple covalent bond. N + N N≡N or N⋮⋮N In the N2 molecule, there is only one unshared pair of electrons in each nitrogen atom. The HI molecule contains only one single covalent bond. H• + I H―I or H:I There are 2 double covalent bonds in a molecule of CO2. C 2O O═C═O or O::C::O Carbon monoxide has a triple covalent bond. C O C≡O OR C⋮⋮O Bonding Theories According to VSEPR theory, molecules adjust their shapes to keep pairs of valence electrons as far apart as possible. VSEPR – Valence Shell Electron Pair Repulsion A stereoactive set is a shared pair or an unshared pair of electrons around the central atom. The shape of a molecule of CO2 is linear. The shape of a molecule of HCN is linear. The shape of a molecule of CH4 is tetrahedral. The shape of a molecule of NH3 is trigonal pyramidal. The shape of a molecule of H2O is bent. According to VSEPR theory repulsive forces between unshared pairs of electrons causes water molecules to have their shape. Bond angle = 109.5 degrees Example: CH4 Bond angle = 106.5 degrees Example: NH3 Bond angle = 104.5 degrees Example: H2O Bond angle = 120 degrees Example: CO32- Bond angle = 118.6 degrees Example: O3 Bond angle = 180 degrees Example: CO2 Intermolecular Forces Intermolecular forces – forces between 2 molecules Van der Waals forces - weakest attractions between molecules Dipole interactions – polar molecules attracted to one another Dispersion forces – caused by the motion of electrons Hydrogen bonds – strongest intermolecular forces Hydrogen covalently bonded to a very electronegative atom is also bonded to an unshared electron pair of another electronegative atom. H2O is a polar molecule. 2 H• + O H:O:H
![QUIZ 2: Week of 09.03.12 Name: [7pts] 1.) Thoughtful list of 3](http://s3.studylib.net/store/data/006619037_1-3340fd6e4f1f4575c6d8cf5f79f0ff3e-300x300.png)