VSEPR Theory - bananateachersworld
advertisement
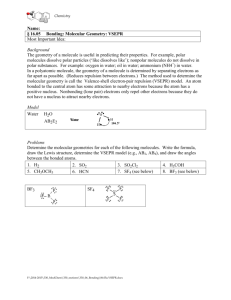
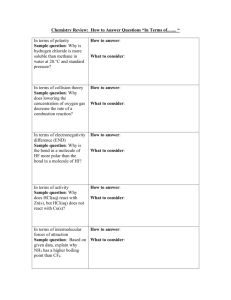
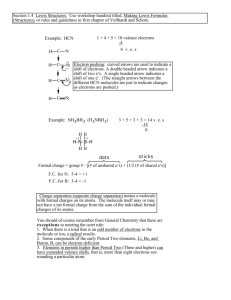
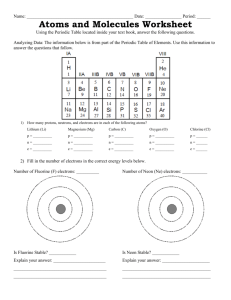
The letters in VSEPR stand for valence shell electron pair repulsion. In 1957, the Canadian chemist R.J. Gillespie, while at McMaster University, developed the VSEPR theory along with a scientist named Nyholm. This theory is used to predict the shapes of molecules. The main proponent of the theory is that the shape of a molecule is determined by the pairs of bonding and nonbonding electrons around the central atom in the molecule. These pairs of electrons will try to stay away from each other in order to minimize all the repulsive forces between them, since electrons are negative and like charges repel. The main concept is to minimize the overall repulsive forces between all pairs of electrons and not just any two pairs of electrons. Bonding pairs and lone pairs of electrons in the valence level of an atom repel one another due to their negative charges. The pairs of electrons take up positions as far from each other as possible about the spherical central atom while remaining in the molecule. The lone pair (LP) will spread more than bonding pair (BP). The order of repulsion can be expressed as follows: LP-LP > LP-BP > BP-BP Do Practice Problems 6 & 7. Page 193