Lecture 3. Fluorescence microscopy I
Letting someone else doing my job
http://probes.invitrogen.com/resources/education/tutorials/1Intro/player.html
Epi-illumination is form of Kohler Illumination:
Objective is also condenser
White light (regular Kohler)
Brightfield, phase, etc
Light is focused
At back aperture
Of the objective,
Conjugate to condenser aperture
Different illumination
And image paths
Lamp or laser lens detector
Detect at 90 degrees
Split with dichroic mirror
Greatly increases S/N
Epi-illumination separates light source,
Fluorescence signal
First barrier filter
Selects excitation
Arc lamp objective lens
Second barrier filter
Selects signal
From background dichroic mirror specimen
S
S
S
2
General Jablonski Diagram
Typical molecular timescales:
Absorption: instantaneous (10 -17 s)
0
1
Fluorescence always from relaxed level of S
1
T
1
Vibronic Relaxation ~ 10 -12 s
Fluorescence: 10 -6 -10 -12 s
10 -9 most typical
Singlet to a singlet (strong)
Phosphorescence: 10 -3 -10 -6 s
Triplet to singlet (weak)
Magnitude of Extinction Coefficients
Extinction coefficient ε:
Beer’s Law A= εcl
Strong absorbers (dyes) have ε between 20,000-100,000
Absorption cross section is also used: 1x 10 -16 cm 2 = 23,000 ε
Brightness= absorption coefficient* QY
Oscillator strength is integral of the absorption band
Sum rule: oscillator strength, f, for one electron over all transitions is:
1x 10 -16 cm 2 eV
Emission spectrum is independent of of path excitation
(both spectral wavelength and width): always from S
1
Emission intensities will be different
Due to different absorption probability:
Franck-Condon Principle
Franck-Condon Principle
Consider electronic states anharmonic oscillators: bond length
Most probable transitions are “vertical”
Big geometry change=broad spectrum (smaller maximum absorption)
Both for absorption and emission: conserve oscillator strength
Geometry and Absorption and Emission Spectra
Bigger Stokes shifts provide better signal to noise
In fluorescence because of filter efficiencies,
(dichroics, low pass, high pass)
But usually involve large geometry change: lower intensities
Spreading out oscillator strength
Fluorescence Quantum Yield φ: important for dyes
Ratio of emitted to absorbed photons
Quantum Yield:
k f k f
k isc
(k is rate,
Inverse of time)
k f
k k f isc
k nonrad
1 k f
k isc
Measured lifetime is sum of natural lifetime and non radiative decay paths
Lifetime is useful contrast
Probe of environment
B. Fluorescent Probes
• Molecular Probes (Invitrogen)
– www.probes.com
– Catalog contains thousands of fluorescent probes, with valuable technical information.
Organelle Probes
Target, Name
Mitochondria
MitoTracker dyes
Excitation Emission
490/578/551 516/599/576
Comments
MT Green accumulates in mitochondria regardless of
MB potential, Red and
Orange in active mitochondria; aldehyde fixable
Golgi
BODIPY FL and TR
C5-ceramide
505 / 589
Lysosomes
LysoTracker
LysoSensor
Endoplasmic reticulum
DiOC6 , DiIC6
Fluorescent Brefeldin A
Various
Various
484 / 549
511, 620
Various
Various
501 / 565
/ 617 TR is better for double labeling, since no green emission
DiO and DiI also stain membranes generally
Inhibitor of protein transport
Immunofluorescence Imaging – Detect Proteins
Fab
IgG
Fc
Fluorescence in situ Hybridization (FISH)
– Detecting Nucleic Acids
Fluorescein: most common dye for microscopy
Blue green
Xanthene family
“green fluorophore” e80,000, φ~0.9
Brightness ~ e f
Many functionalized forms for cell imaging: pH, ion sensing
• High quantum yield
•
General purpose
• But degrades quickly
• Small Stokes shift
(filter bleedthrough)
Rhodamine 6G
Green Red
Xanthene family
“red fluorophore”
Internal Donor-acceptor pair
Red-shifts the spectra relative to fluorescein
Many functionalized forms for cell imaging
• High quantum yield
• General purpose
• Good stability
• Also Small Stokes shift
Green Fluorescent Protein (GFP)
Fluorophore made of
Ser 65 , Tyr 66 and Gly 67
• Requires no co-factor or substrate.
• Works in almost any organism.
• Easy to quantify.
• Genetically modifiable
.
Tsien, Ann.Rev. Biochem. 67 , 509 (1998)
Many fluorescent proteins:
Jellyfish, Coral Reefs
Colored Proteins allow labeling of multiple specific organelles
Variants of Fluorescent Proteins
BFP
EBFP, Sapphire, T-sapphire
CFP
ECFP, mCFP, Cerulean, CyPet, AmCyan, Midoriishi Cyan
GFP
EGFP, Azami Green, TurboGFP, ZsGreen, Emerald
YFP
EYFP, Topaz, Venus, mCitrine, YPet, ZsYellow1, PhiYFP
OFP mBanana, Kusabira Orange, mOrange
RFP dsRed, tangerine, dTamato, mStrawberry, AsRed2, mRFP, mCherry, mRasberry, mPlum, JRed, HcRed
GFP Chromophore
Aequorea : FSYGVQ
Renilla : FSYGDR p-hydroxybenzylidene-imidazolidone
Ser dehydro Tyr - Gly
Chromophore Maturation
Takes ~ 30 min for wild type GFP.
GREEN FLUORESCENCE PROTEIN
Jelly fish isolate DNA encoding GFP
GFP cellular protein couple gene for GFP with gene for protein of interest cellular protein GFP transform cell with altered protein
Completely general and versatile
Problems with Fluorescent Protein
• Size comparable to the target. Might interfere with the function of the target protein
• Maturation time
• Probably not 100% fluorescent
• PH dependence
• Many variants mis-fold when fused to another protein
Linearly Polarized Light
s= horizontal
p= vertical
For propagation
Parallel to floor
Polarizer is device that selects polarization
Can be crystal or film (Polaroid)
Operation of Analyzer (Birefringent)
Light transmitted at angle
relative to angle of 2
Crossed polarizers
I
I
0 cos
2
Combining linear polarized light
IN PHASE
Linear Polarization
OUT OF PHASE
Elliptical Polarization
Circularly Polarized Light
• Decompose to linear polarized light with 1/4
phase shift.
• No direction (always pass 50% through polarizer in dependent of polarizer orientation)
• NOT the same as unpolarized light.
• Can be converted back to linear polarized light with birefringent materials (1/4 wave plate).
Half wave plate rotate the polarization direction of light
Absorption is polarized
Fluorescence is also polarized
GFP Crystal
Anisotropic sample
- Fluorescent intensity is dependent on the polarization _and_ the orientation of the molecules
Isotropic sample
- Fluorescent intensity is independent of excitation polarization
- Fluorescence is polarized if the excitation is polarized.
Fluorescence anisotropy r < 0.4
Microscopic Measurements of
Anisotropy
r = r0 / ( 1 +
/
)
Use Small Numerical Aperture
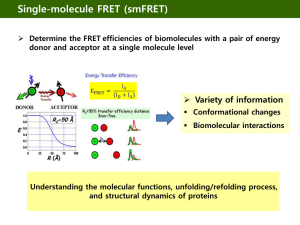
Fluorescence Resonance Energy Transfer (FRET)
Förster Radius
The distance at which energy transfer is
50% efficient (i.e. 50% of excited donors are deactivated by FRET) is defined by the
Förster Radius (R0).
Applications of FRET in Biology
Survey of FRET-Based
Assays
• Protease activity
• Calcium Ion measurements
• cAMP
• Protein tyrosine kinase activity
• Phospholipase C activity
• Protein kinase C activity
• Membrane potential
FRET probes conformational changes
Different conformation gives
Different FRET signature
Inter and Intramolecular
Forms of FRET with
Proteins
CFP-YFP good combo
FRET increases
In both cases
Protein-Protein Interactions
In cytoplasm and membranes
When FRET Occurs
No FRET for
No overlap of donor emission, acceptor absorption
No FRET for
Orthogonal dipole orientation
No FRET for molecules more than 10 nm apart
Number of FRET Publications since 1989
Fluorescence Resonance Energy Transfer -
Detection of Probe Proximity
FRET
F
D
0
F
D
0
F
D
F
A
F
A
Max
F
A
0
F
0
A
0
D
0
D
D
R
0 typically 40-50 Angstroms
50% transfer
FRET
R
6
R
0
6
R
0
6
green
Typical Values of R o
Donor Acceptor R o
(Å)
Fluorescein Tetramethylrhodamine 55
IAEDANS Fluorescein
EDANS DABCYL
46
33
Fluorescein Fluorescein
BODIPY FL BODIPY FL
Fluorescein QSY 7 dye
Cy3 Cy5
CFP YFP
44
57
61
53
50 red
GFPs and other colored “FPs have transformed FRET microscopy
FRET Considerations:
1. Spectral overlap
2. Chromophore orientations
3. Distance dependence (Eff.
1/R 6 )
4. How to quantify?
E
f acceptor f donor
E
f acceptor
f donor f baseline
f
f
A
spillover
D
spillover
Practical Challenges to FRET Quantitation
• Emission from A contaminates D channel (filters)
• Emission from D contaminates A channel
• Unknown labeling levels for D and A
• Signal variation due to bleaching
– Complicates kinetic studies
– Bleaching rate of D can actually be slowed by FRET
Solutions
:
• Separately labeled D and A controls to define bleedthrough
• Acceptor destruction by photobleaching to establish F
D
0
• Dual wavelength ratio imaging to normalize away variations in label levels and bleaching effects
Ca
2+
Release During Shrimp Egg Activation
• From Lindsay et al. (1992). Extracellular Mg 2+ Induces an Intracellular Ca 2+ Wave During Oocyte Activation in the Marine Shrimp Sicyonia ingentis . Dev. Biol. 152:94-
102.
Low quantum yield with no Ca 2+ , big increase
When binds Ca 2+ : up to 50 fold increase
Not absolute concentration of ions, measure relative changes: easier
Fluo- dyes
By Tsien
Choose depending on desired
Range of sensing
Blue Ca
2+
Indicators:
Fluo-3
has single Ex and Em wavelengths
• A visible light excitable dye (488 nm), so Argon laser can be used.
• Emission at 525 nm.
• OK for qualitative detection but not quantitative.
Calcium Sensing Indo-1
Ratiometric using single excitation, dual emission
Excite 338 nm, collect 405, 485 nm fluorescence
Determine absolute calcium concentration by imaging
Free Ca
2+
Concentration in a Purkinje
Neuron from Embryonic Mouse Cerebellum
• Neurons were loaded with fura-2.
• Neurons were stimulated with glutamate receptor agonist.
• The composite image represents the ratio of images obtained with excitation at 340 nm and
380 nm.
Membrane Potential
Membrane Potential Is Due to
Charge Imbalance
Depolarized
Bis-oxonol
Very sensitive: 1%/mV
Resting potential (~-300 mV)
Anionic dye crosses into
Mitochondria when depolarized
(high potassium)
Voltage Sensitive Styryl Chromophore
Fast dye
Stains membrane e
Max~30,000
Large geometry change,
Charge shift upon absorption:
Makes spectra sensitive to electric fields
-
Charge shift in styryl dyes
Excited state
+
+
-
Ground state
E
0
Voltage sensitivity of membrane potential dyes
(electrochromism / Stark Effect)
E
E
0
E
S
1 d d d d intra d extracellular d d d d d
S
0 d d
Hyper-polarized
Red shift
Depolarized
Blue shift h
G
E
E
1
2
G
E
E
2
depends upon dipole moment, polarizability, field strength and orientation
Mechanism of Voltage-dependent
Spectral Shifts in Styryl Dyes -
‘Electrochromism’
Ratiometric approach at
Inflection points for Highest sensitivity
Red shift
Blue shift
1) Single excitation, dual emission:
Laser excitation
2) Dual excitation, same emission:
Arc lamp excitation
F/F: normalizes
Also more sensitive,
Changes are small-10%/100 mV
F/F: normalizes for geometrical factors, bleaching:
Ratiometric approach used for many types of dyes