Lecture 7:
Fluorescence: Polarization and FRET
Bioc 5085
March 31, 2014
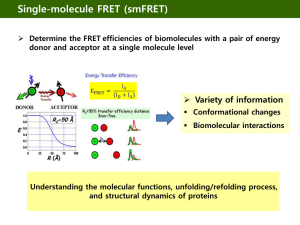
Flourescence Resonance Energy Transfer (FRET)
D = Donor
1 = Absorbance
2 = Emission
A = Acceptor
3 = Absorbance
4 = Emission
FRET occurs and can be readily measured when:
1. Donor (D) has a high quantum yield (f)
2. Donor emission and acceptor (A) absorption spectra overlap (designed by JDA)
3. Donor-acceptor distances are < 1.5 Ro (Ro is known as the Förster distance, see below)
Förster Theory and Förster Distances (Ro)
Förster Distances = Distance between the donor and acceptor at which
energy transfer is (on average) 50% efficient.
According to Förster theory:
Ro6 = (8.79 ´10-25 )k 2h-4fd JDA
8.79 x 10-25 is a combination of several physical constants
2 is the Förster orientation factor
is the refractive index of the medium (typically 1.4 for proteins)
d is the donor fluorescence quantum yield
JDA is the Förster overlap integral
All of the above can be determined experimentally, except for
is indeterminate since it depends not only on the orientation of the
donor and acceptor dipoles, but also on the dynamics of these relative to
one another
is theoretically 2/3 when the donor and acceptor fluorophores reorient
isotropically relative to one another; this has been found to realized in
most cases for probes that are not greatly restricted
FRET transfer efficiencies can be used to measure distances
Importance of FRET to biochemistry is that the transfer efficiency, E, is
a function of the separation of the fluors. Together with the known
Ro, E can be used to measure molecular distances:
E=
R o6
R o6 +R 6
or R = Ro
( )
1-E
E
1
6
Measurement of Transfer Efficiencies
Measure Donor Emission
Intensity (2) in the Presence
and Absence of the Acceptor
E = 1- FA FNo _ A
FRET Donor-Acceptor Pairs: Trp as a Donor
Wu & Brand, Anal. Biochem., 218, 1-13 (1994)
FRET Donor-Acceptor Pairs: “Attached” Donors and
Acceptors
Wu & Brand, Anal. Biochem., 218, 1-13 (1994)
Applications of FRET
Wu & Brand, Anal. Biochem., 218, 1-13 (1994)
Fluorescence Polarization (FP)
Polarizers transmit light that
is either plane
polarized along y or z (can
usually be adjusted
back-and-forth between
these two positions).
Fluorescence Polarization (FP)
FP is based on selectively exciting molecules with their absorption
transition moments (or equivalently absorption dipole) aligned parallel
to the electric vector of polarized light (known as photoselection)
I // - I ^
Polarization = P =
I // + I ^
Absorption
Transition
Moment
Angle
Between
Excitation &
Absorption Emission
Dipoles I//
Dipole
Excitation
Source
//
“parallel”
polarizer
Detect
//
//
//
I
P
0°
0
0
0
0°
0°
0°
1
0
0
0
0
1
1
0
-1
Factors that determine the extent of FP
Limiting Values of FP (+1 and -1) will never be obtained experimentally:
1) Assumed all dipoles in sample are identically aligned (not going to
be true for liquid samples).
2) Assumed all molecules are fixed (not going to be true for liquid
samples).
3) Assumed that the absorption and emission dipoles within
fluorophore are collinear (not generally true).
Limiting polarization (Po) for molecules tumbling (isotropically)
in solution is given by the Perrin-Weber Equation (addresses
assumptions 1 and 3):
ö 1
1 5æ
2
= ç
÷+
2
Po 3 è 3cos f -1ø 3
Po = +1 2 for f = 0; Po = -1/3 for f = 90°
= Angle between absorption and emission dipoles
Extent to which molecule reorients relative to its fluorescence lifetime
determines the extent of polarization (addresses second assumption)
1 æ 1 1 öæ t ö 1
hV
= ç - ÷ç1+ ÷ + where f =
P è Po 3 øè f ø 3
RT
= excited state lifetime
= rotational correlation time (i.e. rotational diffusion constant)
= solvent viscosity, V = volume of the fluorophore
R = gas constant, T = temperature
P 1/ hence, decreasing will yield increased P
P hence, increasing will yield increased P
( V and V MW (by Stokes Law), hence P MW)
Factors that effect extent of reorientation
(and hence, extent of polarization)
Figure 2. Simulation of the relationship between
molecular weight (MW) and fluorescence polarization
(P). Simulations are shown for dyes with various
fluorescence lifetimes (): 1 ns (cyanine dyes) in
purple, 4 ns (fluorescein and Alexa Fluor 488 dyes) in
red, 6 ns (some BODIPY dyes) in green and 20 ns
(dansyl dyes) in blue. At MW = 1000, P = 0.167 for
= 1 ns, P = 0.056 for = 4 ns, P = 0.039 for = 6 ns
and P = 0.012 for = 20 ns. Simulations assume Po
(the fundamental polarization) = 0.5 and rigid
attachment of dyes to spherical carriers.
-Polarization increases as the MW increases (or as the solvent
viscosity increases)
-Polarization decreases as the excited state lifetime () increases
FP Applications
http://probes.invitrogen.com/handbook/boxes/1572.html
Green Fluorescent Protein

![(E)-2,3-bis[(trimethylsilyl)ethynyl]but-2-ene-1,4](http://s3.studylib.net/store/data/007490377_1-34604a482216fb5bf96013c9c6b3224f-300x300.png)