Document
advertisement

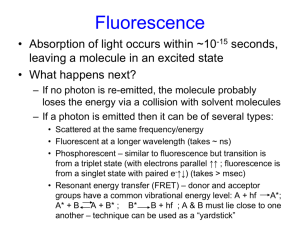
Journal Club: Introduction to Fluorescence Spectroscopy and Microscopy Avtar Singh 4/5/11 Introduction to Journal Club • discuss basic tools / ideas that are useful to all of us • ask questions! • topic-based or article-based? • slides posted on DRBIO site What is Fluorescence? • definition: absorption of light by molecules and subsequent re-emission from excited singlet states Luminescence Photoluminescence Thermoluminescence Chemiluminescence Electroluminescence Bioluminescence etc. Fluorescence Phosphorescence • why useful? • other diagnostic imaging tools for biology: MRI, CT, X-ray, EM • other optical tools: absorption, phase contrast, DIC, scattering (CARS, SRS) • advantages of fluorescence: optical (high-res, in vivo), high contrast, sensitive emission profiles • discovered by Stokes (1852) • initially a nuisance in microscopy: Kohler and Reichert Basic Properties of Light c = 299,792,458 m/s c2 = (ε0μ0)-1 ε0 ≈ 8.85 × 10−12 F·m−1 μ0 = 4π×10 −7 N·A−2 v = c/n Photons = light quanta Interaction of Light with Matter: Dispersion Sellmeier Equation: Transmission Reflection Scattering Absorption Reflection and Transmission: Fresnel Equations θr = θi Incidence = Reflection ni sin θi = nt sin θt Snell’s Law Reflection and Transmission: Brewster’s Angle and TIR Scattering elastic inelastic Quantum Mechanics of the Atom Molecular Orbitals Selection Rules 1. Symmetry: electric dipole moment of the transition must be nonzero 2. Spin: total spin of the system must remain unchanged (photons have no magnetic moment) 3. Nuclear overlap: probability of a transition depends on nuclear overlap integral squared Transition Oscillating Strength Transition Dipole Moment Laporte Symmetry Rule g: σ*, π u: s, p, d, σ, π* (Wiki) Just because the transition is symmetry –forbidden does not mean it can’t occur ignored vibrational motion and used approximate wavefunctions (Cantor and Schimmel p 373) Wigner’s Spin Selection Rule Partial allowance of spin-forbidden transitions due to spin-orbit coupling, typically weak with f ~ 10-7 Nuclear Wavefunctions and Franck-Condon Factors Born-Oppenheimer approximation: In order to simplify the molecular Hamiltonian, assume that the nuclei are stationary (makes sense because they’re much more massive than the electrons) this allows us to separate the nuclear and electron wavefunctions Franck-Condon principle: intensity of a vibronic transition is proportional to the square of the overlap integral between the vibrational wavefunctions of the two states that are involved in the transition Vibronic coupling: in reality, nuclear and electronic motions are coupled can explain some symmetryforbidden transitions that proceed at low f-values Solvent broadening: local solvent environments smear out the vibrational details of absorption / emission spectra Macroscopic Theory of Absorption Beer-Lambert Law Jablonski Diagram Transition Process Timescale (seconds) S(0) S(1) or S(n) Absorption 10-15 S(n) S(1) Internal Conversion 10-14 to 10-10 S(1) S(1) Vibrational Relaxation 10-12 to 10-10 S(1) S(0) Fluorescence 10-9 to 10-7 S(1) T(1) Intersystem Crossing 10-10 to 10-8 S(1) S(0) Non-Radiative Relaxation / Quenching 10-7 to 10-5 T(1) S(0) Phosphorescence 10-3 to 100 ST1) S(0) Non-Radiative Relaxation / Quenching 10-3 to 100 Measuring Fluorescence Fluorescence Saturation Chromophores and Labeling Absorption and Emission Spectra Quantum Yield Fluorescence Lifetime Solvent Effects Quenching Forster Resonance Energy Transfer (FRET) References 1. 2. 3. 4. 5. Lakowicz, Joseph. Principles of Fluorescence Spectroscopy. Springer, 2006. CR Cantor and PR Schimmel. Biophysical Chemistry, Part 2: Techniques for the Study of Biological Structure and Function. WH Freeman and Co., 1980. Olympus microscopy website http://www.olympusmicro.com/primer/techniques/fluorescence/fluorhome.html Warren Zipfel Biomedical Optics Lecture Slides (BME 6260) EPFL Photochemistry Course Lecture Notes http://photochemistry.epfl.ch/PC.html