Flurescence_Microscopy
advertisement

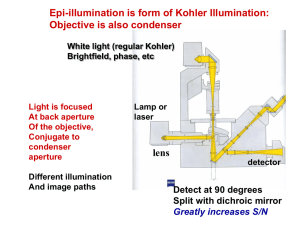
מיקרוסקופיה פלואורסנטית Contrasting techniques - a reminder… • • • • • Brightfield - absorption Darkfield - scattering Phase Contrast - phase interference Polarization Contrast - polarization Differential Interference Contrast (DIC) - polarization + phase interference • Fluorescence Contrast - fluorescence חוב מהשיעור הקודם שיטות להגברת ניגודיות Bright-field Phase-contrast DIC Dark-field Fluorescence techniques • Standard techniques: wide-field confocal 2-photon • Special applications: FRET FLIM FRAP Photoactivation TIRF Fluorescence Fluorescence Excited state excitation emission shorter wavelength, higher energy longer wavelength, less energy Ground state Stoke’s shift Fluorophores (Fluorochromes, chromophores) • Special molecular structure • Aromatic systems (Pi-systems) and metal complexes (with transition metals) • characteristic excitation and emission spectra Fluorophores Filters How can we separate light with specific wavelength from the rest of the light? Filters Filter nomenclature • Excitation filters: x • Emission filters: m • Beamsplitter (dichroic mirror): bs, dc, FT • 480/30 = the center wavelength is at 480nm; full bandwidth is 30 [ = +/- 15] • BP = bandpass, light within the given range of wavelengths passes through (BP 450-490) • LP = indicates a longpass filter which transmits wavelengths longer than the shown number and blocks shorter wavelengths (LP 500) • SP = indicates a shortpass filter which transmits wavelengths shorter than the shown number, and blocks longer wavelengths Filter nomenclature Filters Multiple Band-Pass Filters Basic idea Basic design of epi fluorescence Objective acts as condenser; excitation light reflected away from eyes The cube Excitation/emission spectra always a bit overlapping filterblock has to separate them a) Exitation filter b) Dichroic mirror (beamsplitter) a) Emission filter The cube Excitation / emission excitation and emission spectra of EGFP (green) and Cy5 (blue) excitation and emission spectra of EGFP (green) and Cy2 (blue) No filter can separate these wavelengths! Where to check spectra? You can plot and compare spectra and check spectra compatibility for many fluorophores using the following Spectra Viewers. Invitrogen Data Base BD Fluorescence Spectrum Viewer University of Arizona Data Base Fluorescent Probe Excitation Efficiency (Olympus jave tutorial) Choosing Fluorophore Combinations for Confocal Microscopy (Olympus java tutorial) Photobleaching • Photobleaching - When a fluorophore permanently loses the ability to fluoresce due to photon-induced chemical damage and covalent modification. Photobleaching • At low excitation intensities, pb occurs but at lower rate. • Bleaching is often photodynamic - involves light and oxygen. • Singlet oxygen has a lifetime of ~1 µs and a diffusion coefficient ~10-5 cm2/s. Therefore, potential photodamage radius is ~50 nm. Standard techniques • • • • wide-field Confocal Spinning disk confocal 2-photon Wide-field fluorescence • reflected light method • Multiple wavelength source (polychromatic, i.e. mercury lamp) • Illumination of whole sample Wide-field vs confocal Wide-field image confocal image Molecular probes test slide Nr 4, mouse intestine Point illumination Widefield Illumination Point Illumination Light sources for point illumination Excitation light must be focused to a diffraction limited spot Could be done with an arc lamp and pinhole – but very inefficient Enter the laser: Perfectly collimated and high power Excitation light Objective lens Sample Point illumination Fluorescence Illumination of a single point Camera Tube lens Excitation light Emission light Objective lens Sample Problem – fluorescence is emitted along entire illuminated cone, not just at focus The confocal microscope Detector Pinhole Tube lens Excitation light Emission light Objective lens Sample The confocal microscope • method to get rid of the out of focus light less blur • whole sample illuminated (by scanning single wavelength laser) • only light from the focal plane is passing through the pinhole to the detector Scanning Changing entrance angle of illumination moves illumination spot on sample Objective lens Sample The emission spot moves, so we have to make sure pinhole is coincident with it Improved PSF and pinhole size Why can’t it be as small as possible? • Reduced number of photons that arrive at the detector from the specimen may lead to a reduced signal-to-noise ratio. • Raising the intensity of the excitation light can damage the specimen. • Optical sectioning does not improve considerably with the pinhole size below a limit that approximates the radius of the first zero of the Airy disk. PSF for the focal plane and planes parallel to it: (a) conventional diffraction pattern (b) Confocal case. How big should your pinhole be? • Width of point spread function at pinhole = Airy disk diameter × magnification of lens = 1 Airy unit = resolution of lens × magnification of lens × 2 – 100x / 1.4 NA: resolution = 220nm, so 1 Airy unit = 44 mm – 40x / 1.3 NA: resolution = 235nm, so 1 Airy unit = 19 mm – 20x / 0.75 NA: resolution = 407nm, so 1 Airy unit = 16 mm – 10x / 0.45 NA: resolution = 678nm, so 1 Airy unit = 14 mm How big should your pinhole be? • A pinhole of 1 airy unit (AU) gives the best signal/noise. • A pinhole of 0.5 airy units (AU) will often improve resolution IF THE SIGNAL IS STRONG. Confocal Use: • to reduce blur in the picture high contrast fluorescence pictures (low background) • optical sectioning (without cutting); 3D reassembly possible Careful: increasing image size (more pixels) does not mean that the objective can resolve the same!!! (resolution determined by NA, a property of the objective) Spinning Disk Confocal Spinning Disk • Fast – multiple points are illuminated at once • Photon efficient – high QE of CCD • Gentler on live samples – usually lower laser power • Fixed pinhole • Small field of view (usually) • Crosstalk through adjacent pinholes limits sample thickness Relative Sensitivity • Widefield 100 • Spinning-Disk Confocal 25 • Laser-scanning Confocal 1 • See Murray JM et al, J. Microscopy 2007 vol. 228 p390-405 2-photon microscopy Excited state Excitation: long wavelength (low energy) Emission: shorter wavelength (higher energy) than excitation Each photon gives ½ the required energy Ground state 2-photon microscopy Use of lower energy light to excite the sample (higher wavelength) 1-photon: 488nm 2-photon: 843nm Advantages: IR light penetrates deeper into the tissue than shorter wavelength 2-photon excitation only occurs at the focal plane less bleaching above and below the section Use for deep tissue imaging Special applications: • FRET and FLIM • FRAP/FLIP and photoactivation • TIRF FRET (Fluorescence Resonance Energy Transfer) • method to investigate molecular interactions • Principle: a close acceptor molecule can take the excitation energy from the donor (distance 1-10 nm) No FRET Exited state FRET situation: Excitation of the donor (GFP) but emission comes from the acceptor (RFP) Exited state Energy transfer, no emission! Exited state Donor (GFP) Acceptor (RFP) Ground state Ground state Ground state FRET • Both Acceptor and Donor are fluorescent • The Donor is excited and its emission excites the Acceptor Ex(D) Em(D) Em(A) Ex(A) FRET • FRET is a competing process for the disposition of the energy of a photo excited electron. • Donor emission decreases • Donor lifetime decreases • Acceptor emission increases FRET Energy transfer efficiency • Depends on: Donor emission and acceptor absorption spectra, relative orientation of D and A FRET FRET ways to measure: • Acceptor emission Detect the emission of the acceptor after excitation of the donor, e.g. excite GFP with 488 but detect RFP at 610 (GFP emission at 520) • Donor emission after acceptor bleaching take image of donor, then bleach acceptor (with acceptor excitation wavelength - RFP:580nm), take another image of donor should be brighter! FRET Advantages Disadvantages Cheap implementation Free fluorophors can mask energy transfer high resolution (1-10nm) pH sensitive Living cells Weak effect Real time Location of fluorophors is critical FLIM (Fluorescence Lifetime Imaging Microscopy) • measures the lifetime of the excited state (delay between excitation and emission) • every fluorophore has a unique natural lifetime • lifetime can be changed by the environment, such as: Ion concentration Oxygen concentration ∆t=lifetime pH Protein-protein interactions FLIM - advantages In this method we measure the lifetime of the excited state and not the fluorescence intensity, therefore: • We can separate fluorophores with similar spectra. • We minimize the effect of photon scattering in thick layers of sample. lifetime = ½ of all electrons are fallen back 1/e 1 2 FLIM - Measurement approaches • Frequency domain • Modulated excitation • Lock-in detect emission phase • Time domain (pulsed exc.) • Gated intensifier Photon inefficient • Time-correlated single photon counting Very efficient one photon per pulse slow Time gates FLIM Lifetime histogram Excitation of many electrons at the same time count the different times when they are falling back down (i.e. photons are emitted) decay curve lifetime = ½ of all electrons are fallen back Example of FLIM-FRET measurement GFP expressed in COS 1 cell: average lifetime of 2523 ps fused GFP-RFP expressed in COS 1 cell: average lifetime of 2108 ps Joan Grindlay, R7 FLIM Hepatocyte membrane-stained with NBD, which has a hydrophobicity-dependent lifetime (TCSPC, 3 minutes for 300x300 pixels ) FLIM For FLIM-FRET you still need: a suitable FRET-pair with the right orientation of the π-orbitals Interaction of proteins is not enough, because fluorophores have to be close enough and in the right orientation! Use of FLIM: measurements of concentration changes (Ca+2), pH change etc, Protein interactions Special applications: • FRET and FLIM • FRAP/FLIP and photoactivation • TIRF FRAP (Fluorescence Recovery After Photobleaching) Need: to probe transport Idea: bleach in one area, watch recovery by transport from other areas FRAP Measuring Cdc42 diffusion constant in yeast Result: df = (0.036 ± 0.017) μm2/s Marco et al. 2007 Cell 129:411-422 FRAP • Intense illumination with 405 laser bleaches the sample within the selected region observation of the recovery before 0.65 s 0.78 s Use: to measure the mobility/dynamics of proteins under different conditions FLIP (Fluorescence Loss in Photo-bleaching) Need: probe connectivity Idea: bleach in one compartment, watch loss in connected compartments by exchange Bleach one area repeatedly. Entire ER dims. ER is contiguous Photoactivation (Better?) FRAP/FLIP alternative Some fluorophores can be activated by light • Photo-uncagable dyes • GFP-family proteins Look for weak light against dark background Instead of slight dimming of bright background Activate a small area Watch fluorescence spread photoactivation • Fluorophore only becomes active (= fluorescent) if excited (e.g. with 405 laser) due to structural change Pictures taken from a activation movie: activation of a line trough the lamellipodia of the cell, activated GFP_F diffuses quickly Photoactivation - Proteins Off-On • PA-GFP, PS-CFP Color change • Kaede, KikGR, Eos, • Dendra (activatable by blue) Reversibly Switchable • asCP, KFP (tetrameric) • Dronpa Dendra2 demo green Activate before after red photoactivation Dronpa – photoswitchable on and off Ando et al. 2004, Science 306: 1370-1373 photoactivation Tracking actin flow with Dronpa Kiuchi, T. et al. J. Cell Biol. 2007;177:465-476 Special applications: • FRET and FLIM • FRAP/FLIP and photoactivation • TIRF TIRF (Total Internal Reflection Fluorescence) You need: • TIRF objectives with high NA • TIRF condensor, where you are able to change the angle of illumination • Glass coverslips TIRF micro.magnet.fsu.edu Result: very thin section at the bottom of the sample 150-200nm Use: to study membrane dynamics (endocytosis, focal adhesions, receptor binding) Nikon TE 2000 TIRF vs epi FAK-lasp in epi mode (wide field) FAK-lasp in tirf mode (wide field) Heather Spence, R10 TIRF vs epi Lasp in confocal sectioning Lasp in TIRF mode Heather Spence, R10 Summary/comparison method excitation detection sectioning use Wide field Whole sample Whole sample No sectioning Simple fluorescence samples confocal Whole sample One z-plane 350-500nm High contrast images, optical sectioning 2-Photon One z-plane One z-plane 500-700nm Deep tissue imaging, optical sectioning FRET Protein interactions, small distances FLIM Environmental changes, protein interactions FRAP/FLIP + dynamics/mobility photoactivation TIRF Only bottom plane Only bottom plane 150-200nm Membrane dynamics Light source for fluorescence microscopy Arc lamps Xenon Mercury Laser types UV Argon Blue diode IR 351 364 457 477 488 405 440 Helium-Cadmium 354 Krypton-Argon Green Helium-Neon Yellow Helium-Neon Orange Helium-Neon 514 442 488 569 647 543 594 612 Red Helium-Neon 633 Red diode 635 650 Ti:Sapphire 720-980