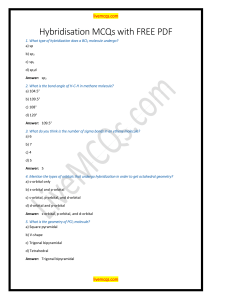
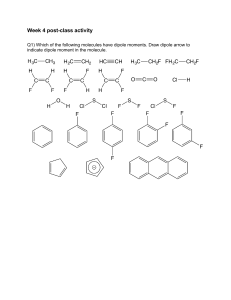
SI Session Chapter 9 April 1st, 2021 1.) Identify the orbitals below, and determine whether a sigma or a pi bond will form: ____ ____ Type of bond formed: _______________________ ____ ____ Type of bond formed: ______________________ _____ _____ Type of bond formed: ______________________ 2.) Predict the likely orbitals involved in the bonding for the following molecules: a.) H2 b.) HF 3.) Based on your understanding of hybridized orbitals, answer the following questions: a.) What is the hybridization around boron in BH3? b.) What type of orbital will the lone pair electrons around nitrogen in NH3 be in? c.) How many sigma bonds are in the molecule CO2? d.) What is the hybridization around carbon is carbon dioxide? 4.) Determine the hybridization around the bolded atoms: a.) H2O b.) NO3- c.) CN- 5.) What is the hybridization around xenon in XeF2?