Hybridization - Teacher Pages
advertisement

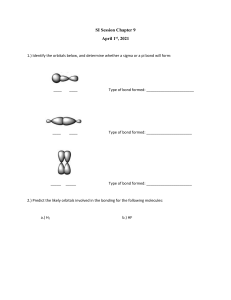
Hybridization This is process in which atomic orbitals are mixed to form new identical hybrid orbitals. Procedure for determining hybridized orbitals. 1. Draw the Lewis Diagram for the molecule. 2. Determine the Stearic Number, which are the groups around the central atom. (A group is another atom sharing electrons or a lone pair). 3. 2 = sp Linear shape 3= sp2 Trigonal Planar 4 = sp3 Tetrahedral 5 = dsp3 Trigonal bipyramidal 6 = d2sp3 Octahedral What is the hybridization around the central atom of the following compounds? BF3 CF4 SO2 CO2 PCl5 Sigma and Pi bondsSigma bond-electrons in an end to end overlapping regions to share. Pi bond- electrons in a parallel overlapping region to share. Single bonds consist of a sigma bond Double bonds consist of a sigma and pi bond Triple bonds consist of a sigma and two pi bonds. 1 2