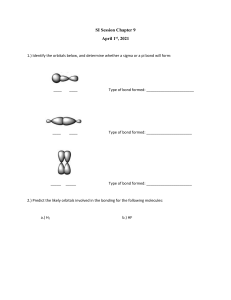
Document 11123961
advertisement
KIJ l
Name:
Se22S Quiz
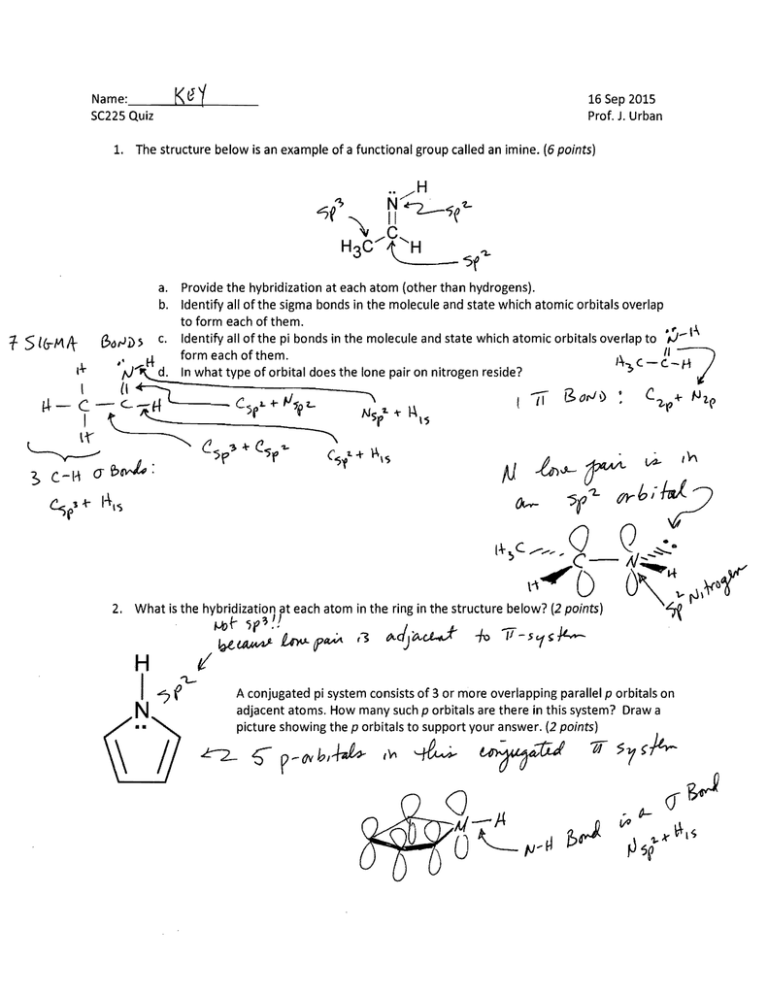
16 Sep 201S Prof. J. Urban 1. The structure below is an example of a functional group called an imine. (6 points)
t S l6-M It
t3>orJ i> ~
"
1+
f.+ l.
a. Provide the hybridization at each atom (other than hydrogens).
b. Identify all of the sigma bonds in the molecule and state which atomic orbitals overlap
to form each of them.
I
I,
• t'
c. Identify all of the pi bonds in the molecule and state which atomic orbitals overlap to ,...) ­
I
C-
t
I
()
,
~
~~:
i.\.,. c
form each of them.
~
\t'.
~
S C-H
H
tJ.. .~.
.
In what type of orbital does the lone pair on nitrogen reside?
{I ~
"\ C.-Ii
- - C:)oJ.. + tJ"iDt... /J
L5p? +
Nsp1..
If
C
'51' -z..
T
1-\,'5
~
C,,;.t + tl.\S
;;1
-
/I
BorJ~'
I'
C
"2-1'+
Ai -tnJ- ~
0<--
-l'--;;J
I
fJ
1.('
~ In
51' '2. (frb i ~
1 +t.C~"''' , Q
c-Q~.
IV-'­
~
2.
\'f~ 0
What is the hybridization at each atom in the ring in the structure below? (2 points)
.
H
IN '? f"-
o
f....br '5(J?!!
~~ ~ (faJA (s I)I.~ "()v~ -fo
-.
Ii - 5 '1 s:
ti\"" ~\\<.r
~
.JL.-.
t!
A conjugated pi system consists of 3 or more overlapping parallel p orbitals on
adjacent atoms. How many such p orbitals are there in this system? Draw a
C-:ic~e ;::n:,~rbi:S
tzrt
yo:;;:ttn~