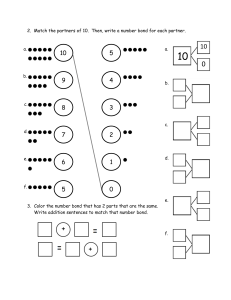
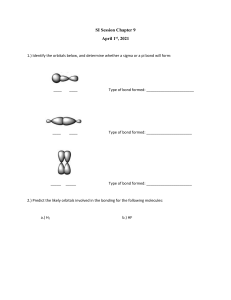
Week 4 post-class activity Q1) Which of the following molecules have dipole moments. Draw dipole arrow to indicate dipole moment in the molecule. Q2) Draw and complete the hybridization scheme of hydrogen cyanide, HCN. Q3) H2SO4 has two Lewis structures as shown below. Experiments have measured S-O bond length in H2SO4. They are S-O single bond = 157 pm and S=O double bonds = 142 pm. Based on the experimental results, which is the preferred structure? Explain your response. Literature bond lengths of S-O and S=O are 158 pm and 143 pm respectively. A B Q4) SO42- has two Lewis structures as shown below. Experiments have shown that all four S - O bond lengths = 147 pm in SO42-. Based on the experimental results, which is the preferred structure? Explain your response. Literature bond lengths of S-O and S=O are 158 pm and 143 pm respectively. C D Q5) Adapted from Blackman Chapter 5 review questions 5.53. Capsaicin is the molecule responsible for the hot spiciness of chillies. (a) How many 𝜋 bonds does capsaicin have? (b) Identify the hybridization state of each of the labelled atoms. (c) What are the bond angles around each of the labelled atoms? (d) Redraw the structure of capsaicin adding the lone pairs. Q6) Adapted from Blackman Chapter 5 review question 5.35. Draw and complete the hybridization scheme of the common solvent acetone, (CH3)2CO.