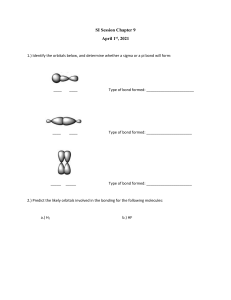
Chapter 9 Paramagnetic means attracted to a magnetic field (magnet) Condition: have unpaired e- (by itself) Ex: So we discuss the molecular orbital model because it is better at explaining the phenomenon above. Hydrogen does NOT hybridize it only uses the s orbital Sigma (single) bond is what is formed with cH4 results from the direct overlap of s and p orbitals. Here we have 4 sigma bonds which are stronger than double and triple bonds A sigma bond: the electron density connecting the atoms is In the middle because of overlapping we use hybridized orbitals Pi bond (double or triple bond): whenever you have a triple/double one is always sigma the other is pi The pie bond is different. The bonding e- are going to be above and below the sigma bond. To make the pi bond we use unhybridized orbitals The unhybridized bond is going to be perpendicular to the sp2 hybrid orbitals but parallel to each other. It is easier to break a pi bond than a sigma bond If triple bond: 1 sigma and 2 pis