Name________________________________ Date__________________ Period____
advertisement

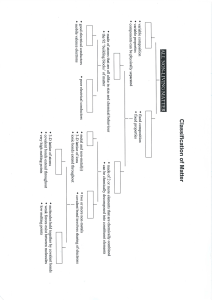
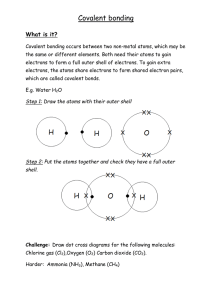
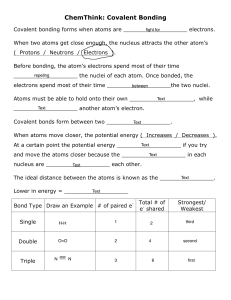
Name________________________________ Date__________________ Period____ Chapter 2, The chemistry of life: Define matter:____________________________________________________________ 1) Atoms are: Subatomic particles: A) Protons B) Neutrons C) Electrons 2) Atoms vs. Isotopes: An isotope of an element differs from an atom in the number of____________. - Radioactive Isotopes: - 3) Elements and Compounds Define element: Define compound: 4) Bonding What is a chemical bond? How are they formed? A) Ionic bonds: Exchange of electrons formation of ions! B) Covalent bonds: Sharing of electron pairs. Single covalent: Double covalent: Triple covalent: C) Van der Waals Forces: In covalent bonds, often the sharing is unequal due to the differenced in the atoms involved. The stronger an atoms pull for electrons the greater the chance the electrons will be located near that atom. Oppositely charged regions on molecules attract one another!