Molecular Compounds Note
advertisement

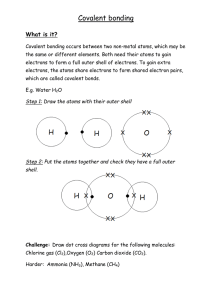
Molecular Compounds and Covalent Bonding A molecular compound is formed between non-metal atoms Since non-metals (found on right side of periodic table) need electrons to become stable, two or more atoms will share its valence electrons to have a full outer shell. A shared pair of valence electrons is called a covalent bond. No transfer of electrons occurs between atoms but rather the sharing. No ions (charged atoms) are formed. Diatomic gases - elements in which two atoms of the same element form a molecule H O Br F I N Cl 2 2 2 2 2 2 2 Example 1: Example 2: hydrogen gas (H2) methane gas (CH4) Example 3: fluorine gas (F2)