Covalent Bonds: Naming & Structure Worksheet
advertisement

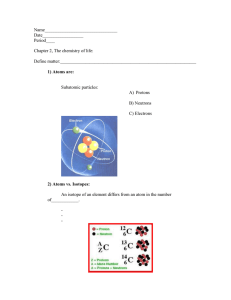
Covalent Bonds Covalent bonds are chemical bonds formed when __________ are __________ between two atoms between _________ _________________ atoms. • Atoms form from Groups 4B (14), 5B (15), 6B (16), and 7B (17). In a fluorine, F2, molecule, each F atom shares one electron and attains an octet. Lone Pair- Valence electrons that are not involved in a chemical bond. Lewis Dot Structure: __________ that show the_____________ between ________ of a molecule and the lone pairs of electrons that may exist in the molecule To name covalent compounds: STEP 1: Name the first nonmetal as an element. STEP 2: Name the second nonmetal with an -ide ending. STEP 3: Use prefixes to indicate the number of atoms (subscript) of each element. To go from name to formula use prefix to give you number! How do you tell of something is an Ionic Bond or Covalent Bond? _________ is between 1 metal and 1 non-metal Covalent is between 2 ______ ___________.