ANSWERS TO WORKBOOK PAGES 17-22 2.1 + 6 n 6 +6 +6 n 7 n 8
advertisement
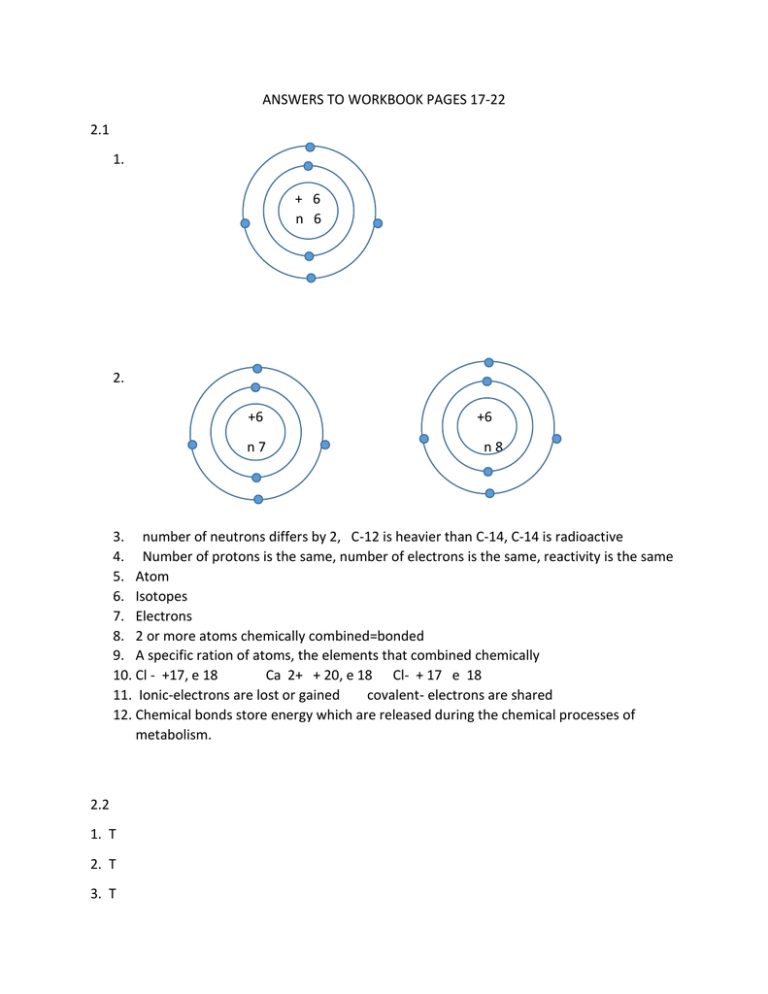
ANSWERS TO WORKBOOK PAGES 17-22 2.1 1. + 6 n 6 2. +6 n7 +6 n8 3. number of neutrons differs by 2, C-12 is heavier than C-14, C-14 is radioactive 4. Number of protons is the same, number of electrons is the same, reactivity is the same 5. Atom 6. Isotopes 7. Electrons 8. 2 or more atoms chemically combined=bonded 9. A specific ration of atoms, the elements that combined chemically 10. Cl - +17, e 18 Ca 2+ + 20, e 18 Cl- + 17 e 18 11. Ionic-electrons are lost or gained covalent- electrons are shared 12. Chemical bonds store energy which are released during the chemical processes of metabolism. 2.2 1. T 2. T 3. T 4. T 5. mixture physical combination of two or more substances cinnamon sugar Solute substance being dissolve salt in water Solvent substance doing the dissolving water Suspension mixture of water and nondissolved substance sand in water Solution homogeneous mixture salt water 6. there is an equal number of H ions as OH ion. 7. the concentration of H ions in solution 8. increasing acidic would go towards the left; increasing basic would go to the right 9. lemon juice, stomach acid 11. add a weak acid to the solution. 12. they help maintain the pH of the system 2.3 1. 4 2. the presence of 4 valence electrons allows carbon to bond in several different ways (single, double, triple bonds) as well as with many different atoms at the same time. 3. macromolecules 4. Dehydration synthesis reactions 5. monomers 6. carbohydrates C,H,O (ratio; 2 H to 1 O ) energy Lipids C<H<O (no ratio) energy Proteins C,H,O,N,S cellular growth/repair Nucleic acids C,H,O,N inheritance,DNA,RNA 7. Organic compounds got their name from the fact that they are all found in living organisms and possess covalent bonds ANSWERS TO TEXTBOOK QUESTIONS Chapter 3 chapter review page 60-61 Multiple choice 1. 2. 3. 4. 5. 6. 7. 8. A B D C A B D D True false 1. 2. 3. 4. 5. 6. 7. 8. T F F F F F T F COVALENT REACTION ATOMIC NUMBER RELEASE VOLUME PROTON Word Relationships 1. 2. 3. 4. 5. Terms related to the nucleus of an atom energy level does not belong All are elements except carbon dioxide which is a compound All are states of matter except mass which is a chemical property Terms associated with the 2nd or 3rd energy level except 2 Terms are associated with covalent bond except electron transfer which deals with ionic bond 6. Terms deal with physical properties except ability to burn which is a chemical property 7. Terms refer to chemical changes except physical change’ 8. Terms associated with the concept of an atom with the exception of divisible particle. Chapter 4 chapter review page 80 Multiple choice 1. 2. 3. 4. 5. 6. 7. 8. B D A B C C A D True false 1. 2. 3. 4. 5. 6. 7. 8. F F T F T T F T SUSPENSION ACIDS MONOSACCHARIDES POLYUNSATURATED WORD RELATIONSHIPS 1. All terms deal with mixtures in which one substance, solute, is dissolved in another substance, solvent with the exception for suspension 2. Terms all deal with complex carbohydrates or polysaccharides except for a monosaccharide 3. All are lipids with the exception of proteins 4. All are concerned with the way in which enzymes work, except for nucleic acid











