Covalent Bonding Notes2
advertisement
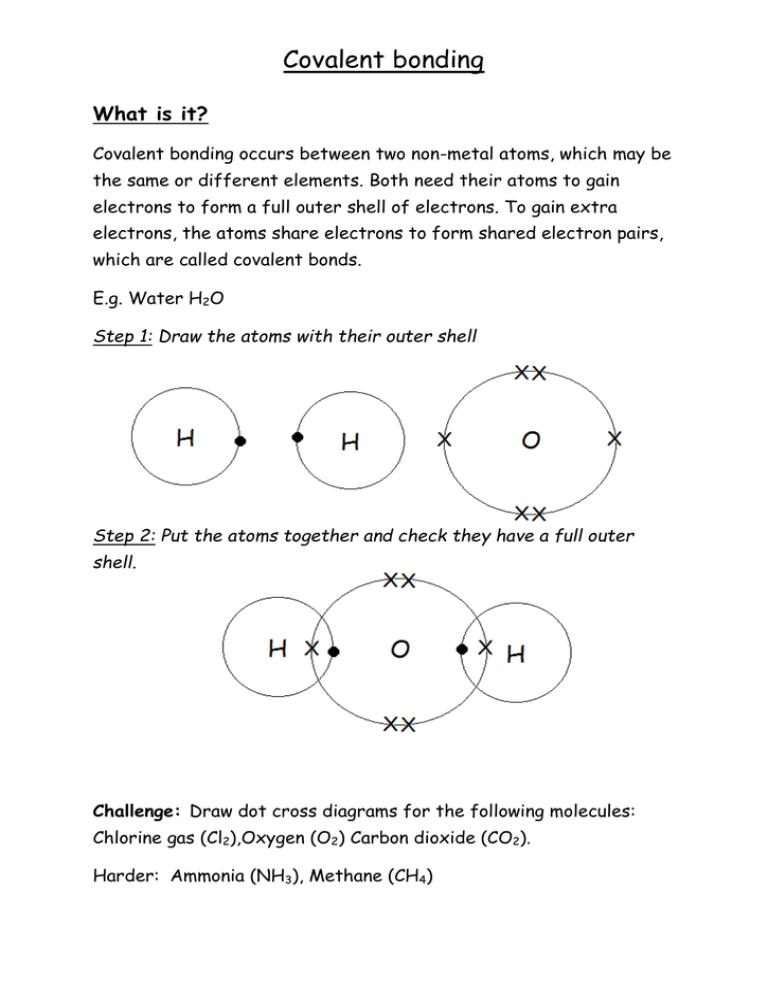
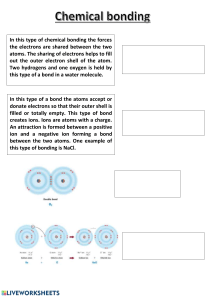
Covalent bonding What is it? Covalent bonding occurs between two non-metal atoms, which may be the same or different elements. Both need their atoms to gain electrons to form a full outer shell of electrons. To gain extra electrons, the atoms share electrons to form shared electron pairs, which are called covalent bonds. E.g. Water H2O Step 1: Draw the atoms with their outer shell Step 2: Put the atoms together and check they have a full outer shell. Challenge: Draw dot cross diagrams for the following molecules: Chlorine gas (Cl2),Oxygen (O2) Carbon dioxide (CO2). Harder: Ammonia (NH3), Methane (CH4)