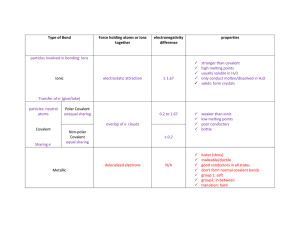
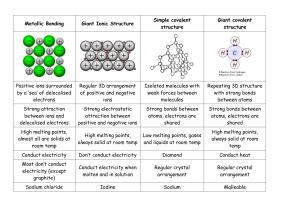
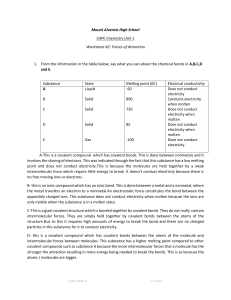
Classification of Matter * fixed composition ¢ fixed properties | | ALL NON-LIVING MATTER ¢ variable composition ¢ variable properties * components can be physically separated ¢ molecules held together by covalent bonds ¢ weak forces exist between molecules ¢ low melting points * two or more non-metals ¢ covalent bond involves sharing of electrons | ¢ made of 2 or more elements that are chemically combined ¢ can be chemically decomposedinto constituent elements ¢ metal and non-metal(s) ¢ 3-D lattice of ions ¢ ionic bonds extend throughout made of atomsthatare all alike in size and chemical behaviour | ¢ poorelectrical conductors ¢ the 92 ‘building blocks’ of matter ¢ goodelectrical conductors ¢ mobile valence electrons ¢ 3-D lattice of atoms ¢ covalent bonds extend throughout ¢ very high melting points