Catalytic Hydrogenation of Alkenes: Relative Stability of Double Bonds
advertisement

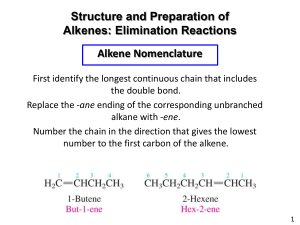
The heat of hydrogenation is a measure of stability. The relative stabilities of related alkenes can be determined by measuring their heats of combustion. The thermodynamic stability of the butenes increases in the order: 1-butene < cis2-butene < trans-2-butene. Highly substituted alkenes are most stable; trans isomers are more stable than cis. The relative stability of the alkenes increases with increasing substitution (hyperconjugation), and trans isomers are usually more stable than cis isomers (crowding). An exception to this stability rule is in medium-ring and smaller cycloalkenes. The trans isomers of cycloalkenes are much more strained than are the corresponding cis isomers. The smallest isolated simple trans cycloalkene is trans-cyclooctene which is 9.2 kcal mol-1 less stable than the cis isomer is very twisted. 11-8 Preparation of Alkenes from Haloalkenes and Alkyl Sulfonates: Bimolecular Elimination Revisited Two approaches to the synthesis of alkenes are elimination reactions and the dehydration of alcohols. Regioselectivity in E2 reactions depends on the base. Haloalkanes (or alkyl sulfonates) in the presence of strong base can undergo elimination of HX with the simultaneous formation of a C=C double bond. In the cases where the hydrogen atom can be removed from more than one carbon atom in the structure, the regioselectivity of the reaction can be controlled to a limited extent. Consider the dehydrobromination of 2-bromo-2-methylbutane. Elimination of HBr proceeds through attack by the base on one of the neighboring hydrogens situated anti to the leaving group. The transition state leading to 2-methyl-2-butene is slightly more stabilized than the one leading to 2-methyl1-butene. The more stable product is formed faster because the structure of the transition state resembles that of the products. Elimination reactions that lead to the more highly substituted alkene are said to follow the Saytzev rule. The double bond preferentially forms between the carbon that contained the leaving group and the most highly substituted adjacent carbon that bears a hydrogen. When a more hindered base is used, more of the thermodynamically less favored terminal alkene is generated. Removal of a secondary hydrogen (C3 in the starting bromide) is sterically more difficult than abstracting a more exposed methyl hydrogen when a hindered base is used. The transition state leading to the more stable product is increased in energy by steric interference with the bulky base. An E2 reaction that generates the thermodynamically less favored isomer is said to follow the Hofmann rule. E2 reactions often favor trans over cis. The E2 reaction can lead to cis/trans alkene mixtures, in some cases with selectivity. This and related reactions appear to be controlled by the relative thermodynamic stabilities of the products. The more stable trans double bond is formed preferentially. Complete selectivity is rare in E2 reactions, however. 11-9 Preparation of Alkenes by Dehydration of Alcohols When alcohols are treated with mineral acid at elevated temperatures, dehydration (E1 or E2) occurs, resulting in alkene formation. As the hydroxy bearing carbon becomes more substituted, the ease of elimination of water increases. Secondary and tertiary alcohols dehydrate by an E1 mechanism. The protonated hydroxy forms an alkyloxonium ion providing a good leaving group: water. Loss of water forms a secondary or tertiary carbocation. Deprotonation forms the alkene. Carbocation side reactions (hydrogen shifts, alkyl shifts, etc.) are possible. The thermodynamically most stable alkene or alkene mixture usually results from unimolecular dehydration in the presence of acid. Whenever possible, the most highly substituted system is generated. Trans-substituted alkenes predominate if there is a choice. Treatment of primary alcohols with mineral acids at high temperatures also leads to alkenes. The reaction of propanol yields propene. The reaction proceeds by protonation of the alcohol, followed by attack by HSO4- or another alcohol molecule (E2 reaction) to remove a proton from one carbon atom and water from the other.