+ 2
advertisement

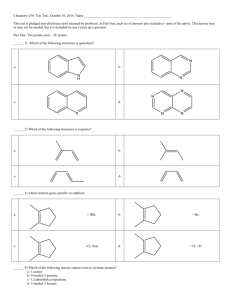
Page 1 of 3 CH235-2F Worksheet 7-Exam 2 October 1, 2014 1. Label 1-6 on the reaction coordinate diagram. Is this an endothermic or exothermic reaction? 1. Potential 2. 3. 4. 5. 6. Energy of the Reactants Energy of Activation Potential Energy of the Transition State Change in Energy from the Transition State to Products Change in Energy from the Reactants to Products (ΔH) Potential Energy of the Products 2. Calculate the index of hydrogen deficiency for the following molecules. 3 2 2 Page 2 of 3 You can use the following formula to calculate the index of hydrogen deficiency: 2(C) + 2 – H – X +N 2 3. Name the following alkenes. (Z)-2-bromo-2-pentene 4-ethyl-1-methylcyclohexene trans-2,5-dimethyl-3-hexene Remember-you must indicate cis/trans or E/Z when possible. 4. Draw the following alkenes. 3-ethyl-5-methyl-3-heptene 4-bromo-1-ethylcyclopentene trans-2-methylhex-3-ene (Z)-2-chloro-2-hexene Page 3 of 3 4. Explain when it is appropriate to use cis/trans and when it is appropriate to use E/Z. When you have a H on either side of the double bond, use cis/trans. If the double bond is tri or tetra substituted (only one side or neither side has a H) then use E/Z. Use Cahn-Ingold Prelog rules to determine the highest priority on each side of the double bond. If the highest priority is on the same side of the double bond, you have Z. If the highest priority is on opposite sides of the double bond, you have E.