Information: The Three Simplest Alkenes
advertisement

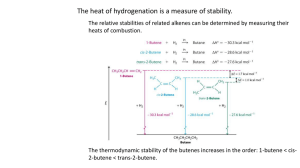
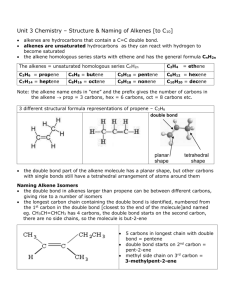
ChemQuest 87 Name: ____________________________ Date: _______________ Hour: _____ Information: The Three Simplest Alkenes H H H H C C C H C H C H H H H H H H C C C C H H H propene H ethene 1-butene H Critical Thinking Questions 1. Observe the three simplest alkenes above. Describe what an alkene is. An alkene is a compound containing only carbon and hydrogen, with a double bonded carbon. 2. Why is there no such thing as "methene"? The prefix “meth” implies one carbon and it would be impossible to have a double bond without two carbon atoms. 3. Below is a structural isomer of the third alkene (above). The name of this isomer is "2butene". H H H C C H H C C H H H a) In 1-butene and in 2-butene, what is the function of the number? It tells which carbon atom has the double bond. b) Why is a number not needed for ethene or propene? There is only one possible position for the double bond. c) Why do you think "3-butene" not a proper name? “3-butene” would be the same compound as 1-butene when rotated in space. 4. What is the bond angle around a carbon with a double bond? Any atom with only 3 electron domains has a 120o bond angle. 5. For an alkene, if there are X carbon atoms, how many hydrogen atoms will there be? 2X 6. Draw 3-octene and 2-methyl-3-octene. Include the hydrogen atoms in your drawing. Information: cis and trans Isomers Note the following isomers of 2-butene and 2-pentene. It is important to know that molecules cannot rotate around a double bond like they can with single bonds. Double bonds are fixed in place and no rotation occurs. H H H H H C C H H C C trans-2-butene H H C H H H C H C H H H H C H C cis-2-butene H C H C H H H H H C C H H C trans-2-pentene H H C C C H H H H C H H H cis-2-pentene Critical Thinking Questions 7. What is the difference between cis and trans isomers? Cis isomers have the branches on the same side of the structures. Trans isomers have their branches on opposite sides of the molecules. 8. In question number three, you drew 3-octene, but you did not know about cis or trans isomers. Now draw cis-3-octene and trans-3-octene. cis trans 9. Why aren't cis or trans designations necessary for propene? Propene doesn’t have two branches to be concerned about. 10. Is trans-3-pentene a proper name for a molecule? Why or why not? No, the name should be trans-2-pentene because “trans-3-pentene” is the exact same molecule as trans-2-pentene and the number designating the double bond should be the lowest possible.