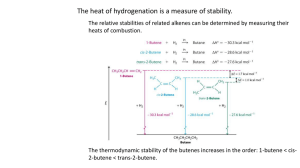
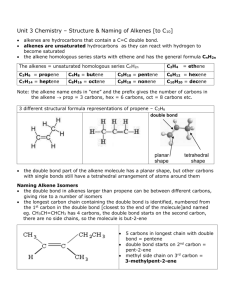
Alkenes Lab
advertisement

Honors Chemistry: Alkenes Exercise Name Topic: Alkenes, Structural Isomers and Organic Nomenclature. Materials: Molecular Model Kits Procedure: You will use a molecular model kit to construct several alkanes. The black balls represent carbon and therefore have 4 holes to form bonds; the white balls represent hydrogen and have 1 hole. Follow the instructions for each part and make sure to have your instructor initial your paper for each of the structures you build. 1. Build and draw dash structures for each of the following: a. propene and propane What similarities and differences can you identify between these two molecules? Try rotating the bonds, what do you notice? b. 2-butene c. 3-methyl-1-pentene 2. Construct, draw dash structures for, and name two structural isomers of C5H10. Honors Chemistry: Alkenes Exercise Name Questions: 1. The molecular formulas for the first few alkenes are as follows: Ethene C2H4 Propene C3H6 Butene C4H8 Think about the relationship between the number of carbons and the number of hydrogens and determine the molecular formula for: a. an alkene with 20 carbon atoms b. an alkene with 100 hydrogen atoms 2. Fill-in the missing spaces in the table below. Name A Dash Line-angle Structure Structure 3-hexene B C 3-ethyl-2-hexene H H H H H C C C C C H H C H H H D H H C H H H