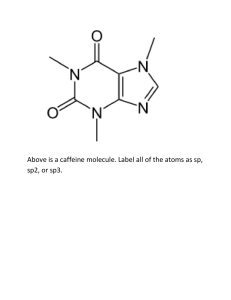
DRAWING STRUCTURES
advertisement
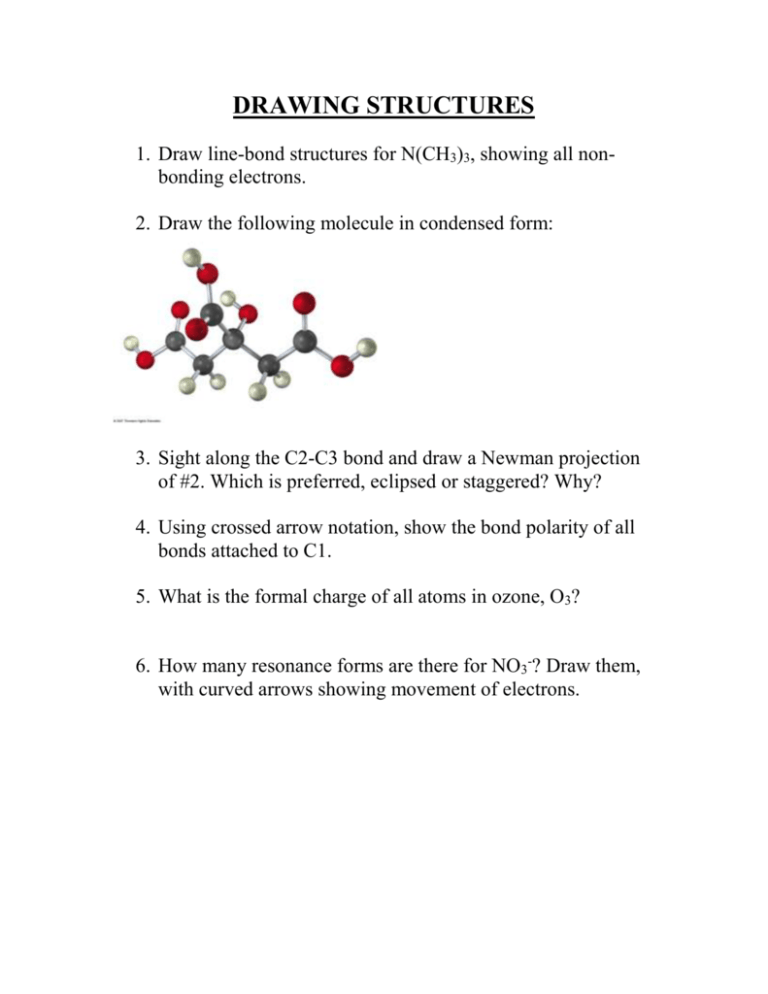
DRAWING STRUCTURES 1. Draw line-bond structures for N(CH3)3, showing all nonbonding electrons. 2. Draw the following molecule in condensed form: 3. Sight along the C2-C3 bond and draw a Newman projection of #2. Which is preferred, eclipsed or staggered? Why? 4. Using crossed arrow notation, show the bond polarity of all bonds attached to C1. 5. What is the formal charge of all atoms in ozone, O3? 6. How many resonance forms are there for NO3-? Draw them, with curved arrows showing movement of electrons. ACIDS AND BASES 1. The amino acid phenylalanine has a pKa = 1.83, and tryptophan has pKa = 2.83. Which is the stronger acid? 2. Ethyne and OH- will they react? 3. In electrostatic potential maps, what do organic acids look like? What do bases look like? Why? 4. What is the Lewis definition of an acid and a base? Give an example of each. 5. Are nucleophiles Lewis acids or bases? Explain. FUNCTIONAL GROUPS 1. Draw an ether. 2. Draw a carboxylic acid. 3. Draw a halide. 4. What non-carbon and non-hydrogen atom does an amine have? 5. Do problem 3.3 on pg 81. ALKANES 1. Draw 2,3-dimethyl-4-ethyloctane 2. Do 3.14 on p. 95 3. Between #1 and #2, which one has a higher melting point? 4. Draw the most stable conformation of 1-fluoropropane, using a Newman projection. CYCLOALKANES 1. Which is more stable, cyclopentane or cyclobutane? Explain. 2. Draw the chair flip of cis-1,4-dimethyl-2-chlorocyclohexane. Calculate the steric strain for both and circle the most stable conformation. Use p 128. 3. Which is more stable, a 1,4-trans substituted cyclohexane or its cis isomer? 4. do 4.23 on page 135. MECHANISMS 1. Name the 4 types of organic reactions. 2. Define an electrophile and give an example of one. 3. Draw the mechanism of propene reacting with acid-catalyzed water using curved arrows. Label the electrophile and nucleophile in each step. Label all formal charges. 4. What determines whether a reaction is spontaneous or not? What variables are involved? 5. Draw an energy diagram of a reaction that is exergonic and there are two transition states. The intermediate is in between the reactants and the products. 6. Define what a carbocation intermediate is. 7. Draw the mechanism of 3-methyl-1-butene reacting with HCl to form 2-chloro-2-methylbutane. What shift happened? 8. Explain Markovnikov’s rule. Give an example. ALKENES 1. How many degrees of unsaturation are there in C9H10Cl3NO2? 2. What does a degree of unsaturation mean? 3. Do 6.4 and 6.6 on p 185. 4. Can BrCH=CHCl have cis/trans isomers? Explain. 5. Do 6.12 on p 191. 6. Do 6.48 on p 216 to practice E, Z designation. Is this stereoisomers, regiochemistry, or constitutional isomers? REACTIONS OF ALKENES AND ALKYNES 1. Look at p 221 and fill in the reagents for each EAR. 2. How might you prepare 3-methyl-4-hexanol? 3. Name the 4 alkyne reactions. 4. I want to make an epoxide in two steps. What should I use? 5. Name all the ways to make an alcohol Markovnikov style. 6. Which reaction uses a bromonium ion? 7. Name all the reactions that result in cis products.