Alkenes: Structure, Preparation, and Reactions
advertisement

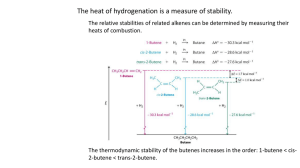
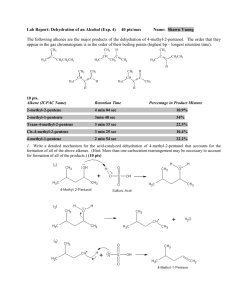
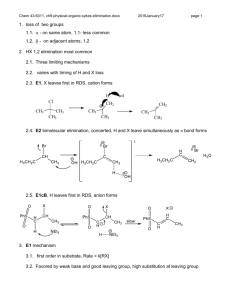
Chapter 5 – Structure and Preparation of Alkenes Double bond - now dealing with sp2 hybrid carbon 5.1 Nomenclature 1-butene 1-hexene Br 2,3-dimethyl-2butene 6-bromo-3-propyl-1-hexene 2-methyl-2-hexene HO 5-methyl-4-hexen-1-ol Common Alkene Substituents H2C C H vinyl CH2=CHCH2 CH2=C CH3 allyl isopropenyl Cycloalkenes Br Cl cyclohexene 1-chlorocyclopentene 3-bromocyclooctene 5.2 Structure and bonding in ethylene Figure 5.1 5.3-5.4 cis-trans isomerism in alkenes 1-butene 2-methylpropene cis-2-butene H H H trans-2-butene Br CH2 CH3 Cl CH 3 O Cinnamaldehyde (trans alkene - E) cis alkene (Z) See Table 5.1 for priority rules Interconversion of cis and trans-2-butene 5.5-5.6 Heats of combustion of isomeric C4H8 alkenes Figure 5.3 5.5-5.6 Heats of combustion of isomeric C4H8 alkenes Figure 5.2 Generally, the more substituted an alkene, the more stable Molecular models of cis-2-butene and trans-2-butene Figure 5.4 5.7 Cycloalkenes - trans not necessarily more stable than cis H H H H C-12 cis and trans ~ equal in energy H H H H H CO2H H Sterculic acid (natural product) 5.8 Preparation of Alkenes - Elimination reactions X C C Y + X Y -C -C 5.9 Dehydration of Alcohols H+ H C C OH + H OH 5.10 Zaitsev Rule Dehydration usually results in more highly substituted alkene being major product - Zaitsev rule (regioselectivity) CH3 OH C CH2CH3 CH3 H2SO4 CH2 80 oC CH2CH3 C + CH3 H3C C CHCH3 H3C 10% HO CH 3 90% CH 3 CH 2 H+ + OH H+ + 5.11 Stereoselectivity in Alcohol Dehydration One stereoisomer is usually favoured in dehydrations H2 SO4, + OH 75% 25% When cis and trans isomers are possible in this reaction the more stable isomer is usually formed in higher yield 5.12 Acid-catalyzed Alcohol Dehydration E1 and E2 E1 OH H2 SO4 + H 2O heat protonation OH 2 deprotonation dissociation H E2 on 1o alcohols – concerted with no cation 5.13 Carbocation Rearrangements in E1 Reactions H3 PO4 OH heat 3% 33% 64% H OH 2 H H Cation rearrangement leads to more stable cation Orbital representation of methyl migration Figure 5.6 5.13 Hydride shifts to more stable carbocations H 2SO4, OH 1- butene 12% H H H H H C C C C H H H H tr ans-2-butene 56% H ci s-2-butene 32% H H H C C C C H H H H H 1o carbocation????? 5.14 Dehydrohalogenation - Elimination with loss of H-X H C C X alkyl halide + NaOCH2CH3 C C base (sodium ethoxide) Cl alkene + HOCH2CH3 + NaX conjugate acid salt NaOCH 2 CH 3 100% H HOCH2 CH3, 55 oC Zaitsev rule followed for regioisomers when a small base such as NaOCH3, NaOCH2CH3 is used. Trans usually favoured over cis. 5.15 The E2 Mechanism - Elimination Bimolecular • Reaction occurs under basic conditions B B H H X X + B-H + X • Reaction is concerted • Rate depends on [base][alkyl halide] i.e. Bimolecular - E2 • C-H bond breaking, C=C bond forming and C-X bond breaking events all occur at the same time The E2 Mechanism - Elimination Bimolecular 5.16 Anti Elimination faster than Syn Elimination Br Br H 500 times faster KOC(CH3) 3 HOC(CH3)3 H 4-ter t -Butylcyclohexene E2 Elimination usually faster when H and leaving group are anti periplanar as opposed to syn periplanar. Conformations of cis- and trans-4-tert-butylcyclohexyl Favourable conformations for fast elimination E2 Elimination usually faster when H and leaving group are anti periplanar as opposed to syn periplanar. Not covering Section 5.17 (Isotope Effects) 5.18 Different Halide Elimination Mechanism - E1 CH3CH2OH + heat Br 2-methyl-1-butene 2-methyl-2-butene 25% 75% CH3CH2OH H H CH3CH2OH R.D.S. is now unimolecular, therefore E1 - usually under neutral or acidic conditions (compare with dehydration earlier)