II. Classification of Matter
advertisement

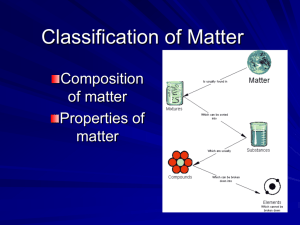
Ch. 1 – Matter and Its Properties Scientific Method Steps Ask a __________________________ Observe and collect data Formulate a hypothesis (a testable if-then statement). The hypothesis serves as a basis for making predictions and for carrying out further experiments. Test your ______________________ – Requires experimentation that provides data to support or refute your hypothesis. Terms to Know Law vs. theory Scientific (natural) _____________: a general statement based on the observed behavior of matter to which no exceptions are known. __________________: a broad generalization that explains a body of facts or phenomena. Quantitative vs. qualitative data Quantitative: numerical (__________________________________) Qualitative: descriptive (___________________________________) Properties & Changes in Matter Extensive vs. Intensive Physical vs. Chemical A. Extensive vs. Intensive Extensive Property depends on the amount of matter present Intensive Property depends on the identity of substance, not the amount A. Extensive vs. Intensive Examples: boiling point intensive volume extensive mass extensive density intensive conductivity intensive B. Physical vs. Chemical Physical Property can be observed without changing the identity of the substance Chemical Property describes the ability of a substance to undergo changes in identity B. Physical vs. Chemical Examples: melting point physical flammable chemical density physical magnetic physical tarnishes in air chemical B. Physical vs. Chemical Physical Change changes the form of a substance without changing its identity properties remain the same B. Physical vs. Chemical Chemical Change changes the identity of a substance products have different properties B. Physical vs. Chemical Signs of a Chemical Change change in color or odor formation of a gas formation of a precipitate (solid) change in light or heat B. Physical vs. Chemical Examples: rusting iron chemical dissolving in water physical burning a log chemical melting ice physical grinding spices physical Ch. 1 - Matter Classification of Matter (p.15-17, 397-398) Matter Flowchart Pure Substances Mixtures A. Matter Flowchart MATTER yes Can it be physically separated? no PURE SUBSTANCE MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no Heterogeneous Mixture Colloids yes Can it be chemically decomposed? Compound Suspensions no Element A. Matter Flowchart Examples: graphite element pepper hetero. mixture sugar (sucrose) compound paint hetero. mixture soda solution B. Pure Substances Element composed of identical atoms EX: copper wire, aluminum foil B. Pure Substances Compound composed of 2 or more elements in a fixed ratio properties differ from those of individual elements EX: table salt (NaCl) B. Pure Substances Law of Definite Composition A given compound always contains the same, fixed ratio of elements. Law of Multiple Proportions Elements can combine in different ratios to form different compounds. B. Pure Substances For example… Two different compounds, each has a definite composition. C. Mixtures Variable combination of 2 or more pure substances. Heterogeneous Homogeneous C. Mixtures Solution homogeneous very small particles no Tyndall effect particles don’t settle EX: rubbing alcohol Tyndall Effect C. Mixtures Colloid heterogeneous medium-sized particles Tyndall effect particles don’t settle EX: milk C. Mixtures Suspension heterogeneous large particles Tyndall effect particles settle EX: fresh-squeezed lemonade C. Mixtures Examples: mayonnaise colloid muddy water suspension fog colloid saltwater solution Italian salad dressing suspension