File
advertisement

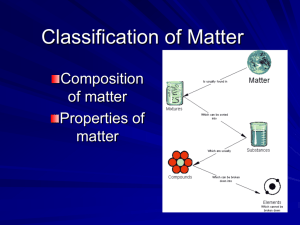
Science is a way to look at the world. A model is a representation that serves to explain a scientific phenomenon. Models are supported by the scientific method which test hypotheses by making predictions about the outcome of an experiment before the experiment is performed. The results provide support or refutation of the hypothesis. Empirical – observable knowledge (operational) Theoretical – explains or describes scientific observations, not observable. (conceptual) Indirect form of knowledge that builds on a concept to further describe or explain an observation. Qualitative Observation describes qualities of matter or changes in matter. changes in color, odor, state. Example: Mg is an odorless, silver-gray solid that burns with bright white light. Quantitative observation Involves a quantity of matter that can be measured. mass volume number of moles Example A 5 cm strip of Mg ribbon burned for 3 s leaving 2 mm of ribbon in the tongs. The study of substances, their structure, their properties, and the changes that can occur to substances. Physical properties – describe the physical appearance and composition of a substance. E.g., color, hardness, texture, phases, density, ability to conduct heat, electricity. Physical Change – a change in size, shape or state. It still retains all the same physical properties. E.g., Melting Ice Heat heat Physical Properties Description Boiling Point Temperature at boiling Point Melting point Temperature at melting Point Malleability Ability to beat or roll a material Ductility Ability to be drawn into a wire. Color Color State Solid, liquid or gas Solubility Ability to dissolve Crystal formation Crystalline appearance Conductivity Ability to transmit heat or electricity Magnetism Magnetic attraction Chemical Properties - describes the reactivity of a substance E.g., ability to burn, flash point, reactions with water, air, acids, heat and litmus paper Chemical Change – a substance changes from one type of matter to another. Ca O S2 Ca S O2 Chemical Property Description Ability to burn Combustion (flame, heat light) Flash point Behavior in air Temperature required to ignite flame Degrade, react or tarnish Reaction in water Tendency to corrode or dissolve Reaction in acids Corrosion, bubble formation Reaction to heat Tendency to melt or decompose Reaction to blue or red litmus Red-acid, blue-base Note: in both Physical and Chemical changes there is Conservation of Mass. anything that has mass and occupies space. STUDYING THE CHANGES TO MATTER IS THE ESSENSE OF CHEMISTRY! A chemist is most interested in the structure and properties of matter and the changes that occur in matter. EXAMPLES: 1. Digestion – biochemistry 2. Oil or Gas plastics – petrochemical engineering 3. Corrosion – chemical engineer 4. Pollution - environmental biologist MATTER MIXTURES PURE SUBSTANCES Contains two or more substances Contains one kind of matter Heterogeneous Homogeneous Mixture where components are intact and visible Mixtures where 2 or more components are dissolved and appear Only one type of atom that cannot as one be broken down Colloid Suspension Mechanical Mixtures ELEMENTS COMPOUNDS -contains two or more elements in a definite fixed proportion -cannot be separated by physical means Solutions APPEAR AS ONE SUBSTANCE PROPERTIES ARE VARIABLE PROPERTIES ARE SAME Pure Substances All particles of the same substance are the same (all particles have the same chemical and physical properties) 2 types of Pure substance Element – all of these are found on the periodic table. (e.g., sulfur) Compound – 2 or more chemically combined elements (e.g.,. CO2, MgCl2) **Note the 2 different types of chemical bonding. Mixtures Combination of pure substances (not chemically combined) 4 types 1. Solutions – homogenous mixture (same parts) Looks similar throughout Soft drinks (sugar is dissolved) 2. Mechanical Mixture – (heterogeneous) You can see all parts clearly E.g.. soil *Note the different parts 3. Suspension – (heterogeneous) The parts are in different states E.g., Mud – soil (solid) & water (liquid) 4. Colloid -(heterogeneous) Similar to suspension but not easy to separate. Colloids look a lot like solutions. If you filter both (solution and colloid) a solution will pass through but a colloid will leave behind chunks if one of the phases was a solid. E.g., Milk, paint, ink… Filtration separates a liquid from a solid (mechanical mixture). Distillation separates a solution (homogeneous mixture). (compound) (element) (element) Electrolysis breaks a compound apart. Chemical Reactions Characteristics of a chemical reaction include: 1. The production of a new substance with UNIQUE physical and chemical properties. 1. A gain or release of energy And sometimes: A phase change such as the formation of a gas (bubbles) or of a solid (precipitate). • All reactions produce a new substance with new physical and chemical properties. • All involve the release or absorption of energy. Heat (thermal energy moving) exits the system – Exothermic Heat enters the system Endothermic Some chemical reactions have phase changes: Bubbles (gas forming) Precipitates (solid forming) Cloudiness (solid forming just not falling) • • Questions #1-9 & 11 (pg. 17 textbook) Read Lab A-3.