Properties of Matter: Classifying Substances & Mixtures
advertisement

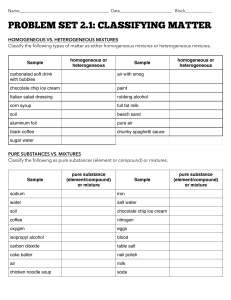
Chapter 2 Properties of Matter Classifying Matter Composition: Latin for “putting together” Pure Substance: matter with uniform composition Elements Cannot be broken down into simpler substances Made up of alike atoms which are the building blocks of matter No two elements can have the same type of atom Examples: aluminum (Al), gold (Au), carbon (C) Compounds Made up of two or more elements joined in a fixed proportion Represented by chemical formulas Ex: H2O, NaCl, CO2, Mixtures Two or more substances mixed together but not chemically combined Composition is not fixed Heterogeneous: parts are noticeably different than one another Cereal, Sand, Homogeneous: Evenly distributed particles and hard to differentiate Water, Stainless steel Classification of mixtures Solutions: Homogeneous substance that dissolves uniformly Ex: Pop, Lemonade, Gatorade Colloids Homogeneous mixture that is mixed together but not dissolved Appears cloudy and scatters light Milk, Cool Whip, Lotions Classification of mixtures Suspensions: Heterogeneous that suspends over time Lake water Composition of Matter Matter Pure Substance Element Compound Mixture Homogeneous Solution Colliod Heterogeneous Suspension