Ch. 17 Review
advertisement

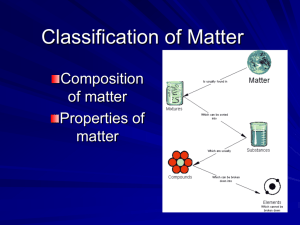
Chapter 17 Study Guide Matter Matter is classified as substances and mixtures. Matter has mass and takes up space Mixture – composition variable, can be separated by a physical means Element – composition definite Compound – two or more elements combined Pure Substances Element matter composed of identical atoms All atoms are the same EX: copper Classifying Matter Colloid vs. Solution-pass a beam of light through the mixture If the beam is invisible- it is a solution If the beam is visible- it is a colloid The visible beam through the colloid is called the Tyndall effect Suspension- heterogeneous mixture containing a liquid in which visible particles settle out over time – like dirty water and Italian salad dressing Mixtures Variable combination of 2 or more pure substances. Homogeneous Mixture (AKA -Solution) even distribution of components very small particles particles never settle EX: saline solution, fresh pickle juice Mixtures Heterogeneous Mixture uneven distribution of components colloids and suspensions EX: granite Pure Substances Compound matter composed of 2 or more elements in a fixed ratio properties differ from those of individual elements NOT a mixture EX: salt (NaCl) Mixtures Colloid medium-sized particles Tyndall effect - particles scatter light (looks cloudy) particles never settle EX: milk, fog Tyndall Effect Because of the Tyndall effect, A light beam is Scattered by the Colloid suspension On the right, but Passes invisibly Through the solution On the left. Physical Properties Physical Property- any characteristic of a material that you can observe or attempt to observe without changing the identity of the substance For example: color, shape, size, melting point, and boiling point, how it flows. Metallic aluminum: Silvery color, easily formed, reflects heat. Behavior of substances- magnetism, ductility- ability of metal to be drawn into wires; malleability- ability of metal to be shaped- pounded into sheets; ability to flow Physical Change A change in the form of a substance without changing its identity. properties remain the same reversible can be used to separate mixtures EX: dissolving, grinding, distillation, mixing sugar and salt Physical Changes Distillation- separating a solution of two liquids through evaporating a liquid and re-condensing its vapor. Vapors from the liquid with the lowest boiling point form first and are condensed and collected Chemical Property A characteristic that indicates whether a substance can undergo a specific chemical change. EX: flammability, reactivity, resistance of a diamond to corrosion Chemical changes can be used to separate substances- done in labs- metals can be removed from ore this way Chemical and Physical Weathering WeatheringPhysical weathering when rocks split as water freezes or as erosion occurs Chemical weathering when acidic water reacts with limestone and results in a new substance that dissolves in water and washes away. Law of Conservation of Mass Mass cannot be created or destroyed Burning a log seems to make mass disappear “missing” mass is actually present in the gases that are produced as the log burns When the log burns new substances are formed. EX: Iron and oxygen combining to form rust, the masses will still be equal.