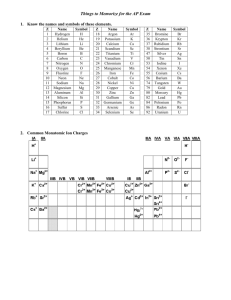
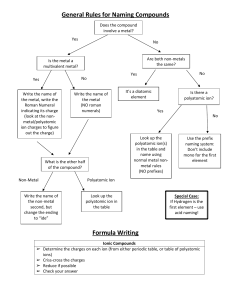
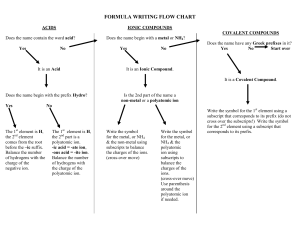
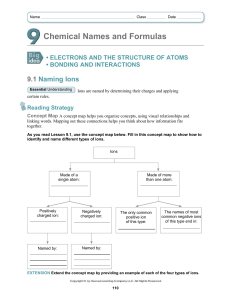
Summary: “Given Chemical Formula, What is the Name?”
advertisement

Summary: “Given Chemical Formula, What is the Name?” Examine Chemical Formula Nonmetal + Nonmetal? Metal + Nonmetal? Formula begins with H and ends with “(aq)”? Molecular (Covalent) Naming Name according to ionic rules… Acid Naming Is it a common chemical (i.e. NH3, H2O)? How many different elements? Just 2 Binary Acid Rules At least 3, one of which is Oxygen No… Yes! Is it a diatomic molecule? (HON F. BrICl) Use Common Name i.e. ammonia Oxyacid Rules No… Is H the first atom in the formula? “hydro” + second element root +“ic” acid Derive name from polyatomic ion with suffix* (i.e. “-ic”) + “acid” i.e. HCl(aq) is “hydrochloric acid” i.e. H2SO4(aq) is “sulfuric acid” * If polyatomic ion ended in –ate, replace it with –ic If polyatomic ion ended in –ite, replace it with –ous Yes! No Prefixes – name is just “Hydrogen _____ide” Yes! Name is the element i.e. F2 = fluorine molecule No… Name using prefix according to the number of each atom *(omit “mono” for first part of name!)* Summary: “Given Name, What is the Chemical Formula?” Does name end with “acid”? Yes! No… Does it start with “hydro”? Is the name just an element? Yes! Yes! It’s a diatomic molecule… No… Identify ions, use cross-over method (cation will always be H+). Add (aq). No… Is it a common name? i.e. water, methane, etc. Yes! Well then, good thing you memorized the formula! No… Does the first part of the name end with “–ic” or “–ous”? Yes! No… Identify ions and use cross-over method (ions will always be H+ and some polyatomic ion). Add (aq) Does the name start with “hydrogen”? Yes! Identify ions, then use crossover method No… Are there prefixes in the name? i.e. mono, di, etc… No… Yes! Use prefixes to tell you the number of atoms of each element (molecular)