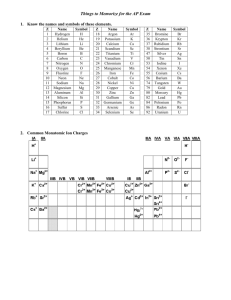
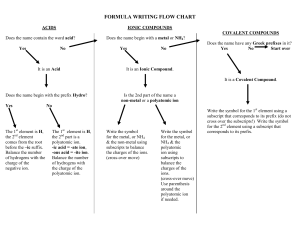
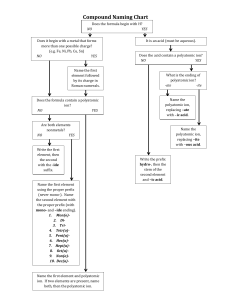
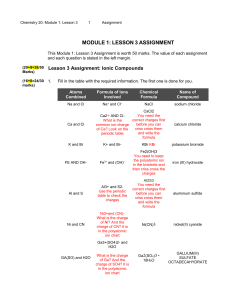
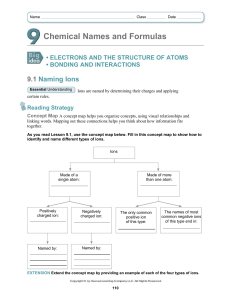
Name: _______________________________________ Date:__________________ Formulas with Polyatomic Ions Instructions: Using the criss-cross method, write the formula for each ionic compound using the charges given. Write all polyatomic ions in a different color. Do not write the parentheses or outside subscript in this color. Example: Co₂( CO₃ )₃ Ask yourself: “How many of each ion do I need for the total charge to equal zero?” That is what the subscript will tell you when looking at the formula. OH⁻ Na⁺ Mg ²⁺ NH₄ ⁺ Ca²⁺ K⁺ Al ³⁺ Pb ⁴⁺ NO₃⁻ CO₃²⁻ SO₄²⁻ PO₄³⁻ Name: _______________________________________ Date:__________________ Example of the criss-cross method: After you find the charges of each ion: *Remember: The subscript is telling you how many of each ion there is. You do NOT need to bring the + or – down with the number.