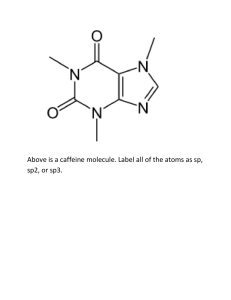
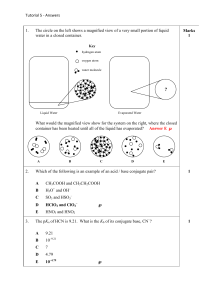
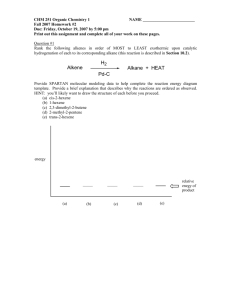
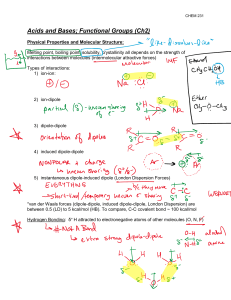
Week 3
advertisement

1) Rank compounds A through D from least to most acidic. 2) Given the following pKa values for the following compounds, rank their conjugate bases (A – D) from least to most basic. pKa 25 pKa 38 pKa 18 pKa 16 A B C D 3) In each reaction, identify the Lewis acid (LA) and Lewis base (LB) on the left side of the reaction. Write LA or LB in the boxes below. 4) Circle the compound that has the most acidic proton (boxed) (Hint: evaluate the stability of the conjugate base). Draw the conjugate base of each acid in the box below it. If the conjugate base can form a resonance structure, draw a resonance structure in the box below it. If no resonance structure of the conjugate base (C.B.) can be drawn, then draw an X in the box. Example 5) For each compound CIRCLE the most acidic proton designated with an arrow. 6) Provide the IUPAC systematic name for the compounds. name this WITHOUT using the 'ISO' prefix for any substituent name 7) Draw the structures. 2-methylheptane 4-ethyl-3,4-dimethyloctane 8) Use the correct chair conformation below to show the most stable conformer of cis-1-isopropyl-2methylcyclohexane. Circle the MOST STABLE conformation below.