Feb 19th SI
advertisement

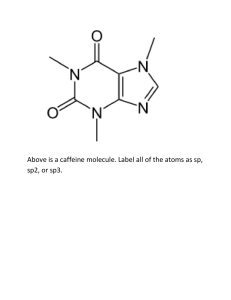
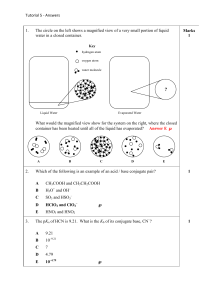
SI Chem 178 Ch 16 (Acid/Base Equilibrium) Feb 19, 2013 Leader: Emily Suggested probs for Fri: 76, 83, 86, 90, 97, 100, 114 A good prob for review: 16.62 Review: What is true of the concentrations of a dissociated monoprotic acid’s proton and conjugate base? How does this help with/relate to calculations? How does this change with di/polyprotic acids? 16.59 b/c only - You more practice if you b. What is the pH of chromate ion? (HCrO4 Deg C. can do “a” on your own for like : ) a solution of 0.100M hydrogen -2) with a Ka of 3*10^-7 @25 c. What is the pH of a 0.120M solution of pyridine? (a base with the chemical formula C5H5N, and a Kb of 1.7*10^-9) New Concepts: Dilution of reactants- Our old buddy Le Chatelier returns! … in an HX H+ + A- equilibrium reaction, which direction will the reaction go if we… Dilute the solution? (What happens in this case overall?) Concentrate the solution? (What happens to my % ionization here?) What previous Concept of Le Chatelier is this similar to? Ka x OR + Kb = Kw = _______ (For a acid conjugate pair) pKa + OR x pKb = pKw = _______ Deg C) “ “ (at 25 What does that “p” stand for? ___________ A smaller pKa means a stronger/weaker acid? How can we reason through this if we didn’t know the answer? If Ka for benzoic acid is 6.3*10^-5, what is its Kb? Is there more than one equation we can use for this? What are the three things we have discussed that affect acid strength? Describe their directional relationships on the PT. 1 2 3 Strength comparisons….. HI >/< HF . . . Why? HClO2 >/< HClO4 . . . Why? CH4 >/< than H2O . . . Why? H2SO4 </> than H2CO3 . . . Why? H2CO3 >/< than H2NO3 . . . Why?