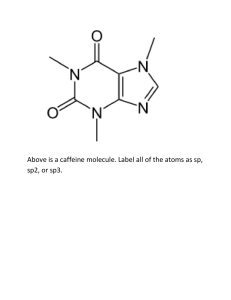
Q3 Summer 1 2015
advertisement
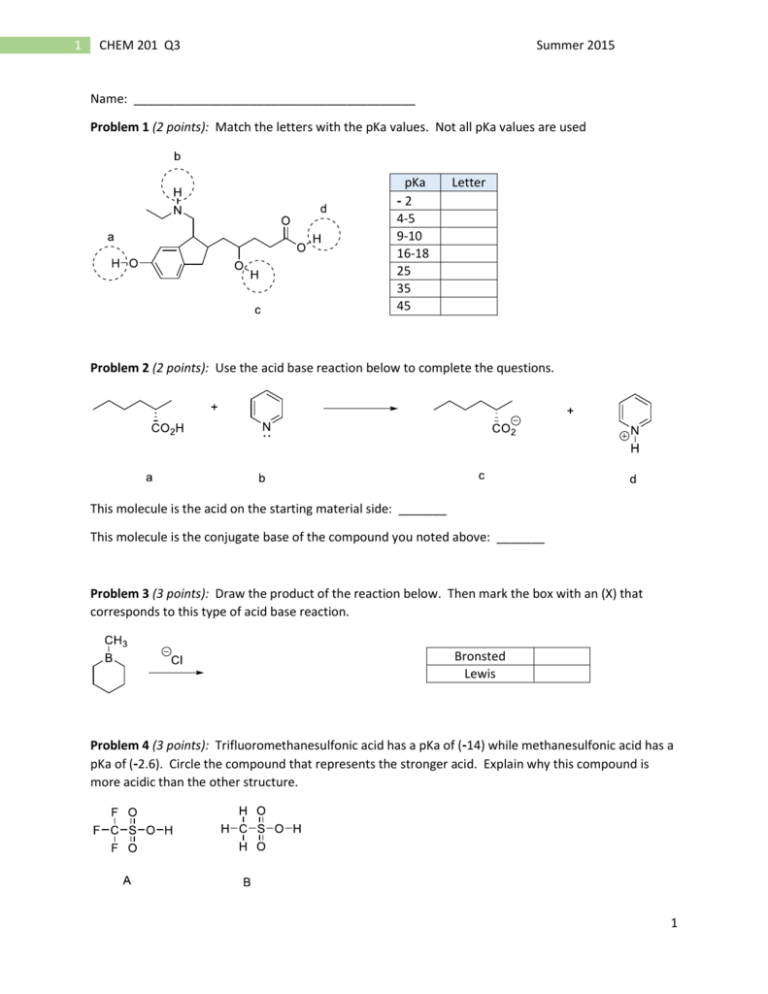
1 CHEM 201 Q3 Summer 2015 Name: _________________________________________ Problem 1 (2 points): Match the letters with the pKa values. Not all pKa values are used pKa Letter -2 4-5 9-10 16-18 25 35 45 Problem 2 (2 points): Use the acid base reaction below to complete the questions. This molecule is the acid on the starting material side: _______ This molecule is the conjugate base of the compound you noted above: _______ Problem 3 (3 points): Draw the product of the reaction below. Then mark the box with an (X) that corresponds to this type of acid base reaction. Bronsted Lewis Problem 4 (3 points): Trifluoromethanesulfonic acid has a pKa of (-14) while methanesulfonic acid has a pKa of (-2.6). Circle the compound that represents the stronger acid. Explain why this compound is more acidic than the other structure. 1 2 CHEM 201 Q3 Summer 2015 Problem 5 (6 points): Draw the conjugate base for each acid. Of the four structures you have drawn, circle the strongest base. Problem 6 (4 points): Draw the product(s) for each acid base reaction. 2