Unit 7 Group Work – Moles, Molecular Mass and Empirical Formulas
advertisement

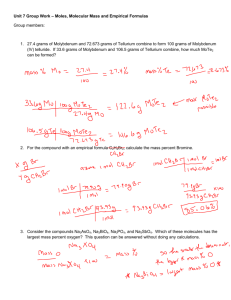
Unit 7 Group Work – Moles, Molecular Mass and Empirical Formulas Group members: 1. 27.4 grams of Molybdenum and 72.673 grams of Tellurium combine to form 100 grams of Molybdenum (IV) telluride. If 33.6 grams of Molybdenum and 106.5 grams of Tellurium combine, how much MoTe2 can be formed? 2. For the compound with an empirical formula CH2Br, calculate the mass percent Bromine. 3. Consider the compounds Na3AsO4, Na3BiO4, Na3PO4, and Na3SbO4. Which of these molecules has the largest mass percent oxygen? This question can be answered without doing any calculations. 4. A compound that has an Empirical Formula of CH2Br has a molar mass of 2.254 kg mol-1: a. What is the molecular formula of this compound? b. What is the mass percent of Bromine? c. How many Bromine atoms would be needed to make 13 g of this molecule? 5. For each of the following, determine the empirical formula: C7H42O14 C0.125H0.5 H0.1667C0.1667O0.5 H0.0179N0.0357O0.05357 6. 25 g of cholesterol contains 20.9676 g of Carbon, 2.9979 g of Hydrogen, and 1.03449 g of Oxygen and has a molar mass of 386.668 g mol-1. a. How many carbon atoms are present in 25 g of cholesterol? b. What is the mass percent of oxygen in cholesterol? c. Please determine the molecular formula of cholesterol.