Name_______________________ Empirical Formula Notes When
advertisement

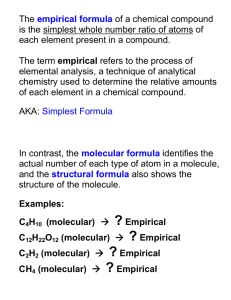
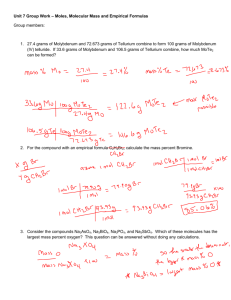
Name_______________________ Empirical Formula Notes When discovering ________________, scientists don’t know what the _____________ of a compound is until they get the _____________ of ___________ of each element. From there, they can determine its _______________________. Empirical formulas: a ratio (determined by __________________ and ______________) to get the _____________ whole number ratio to make a ____________________. What if a substance contains 36.84% nitrogen and 63.16% oxygen, what is the empirical formula? Assuming that you had 100 grams of this substance, then it would be 36.84 grams of nitrogen and 63.16 grams of oxygen? First: ___________________________________________________________________________________________ Nitrogen- grams to moles Oxygen- grams to moles We just figured out the ratio (moles) within the given amounts. But now we need to get the ratio between each element Second: ___________________________________________________________________________________________ What if a substance contains 35.98% aluminum and 64.02% sulfur, what is the empirical formula? What if a substance contains 81.82% carbon and 18.18% hydrogen, what is the empirical formula? Empirical Formula w.s #1 A compound contained 92.25% carbon and 7.75% hydrogen. What is the empirical formula of the compound? Vanadium oxide is used as an industrial catalyst. The percent composition of this oxide is 56.0% vanadium and 44.0% oxygen. Determine the empirical formula for vanadium oxide. While trace impurities of iron and chromium in natural corundum form the gemstones ruby and sapphire, they are basically a binary compound of aluminum and oxygen, with 52.9% Al. Find the empirical formula and give the chemical name for corundum Analysis of a compound containing chlorine and lead reveals that the compound is 59.37% lead. What is the empirical formula and name of the compound?