Exam 2 110 Summer 2014 v2
advertisement
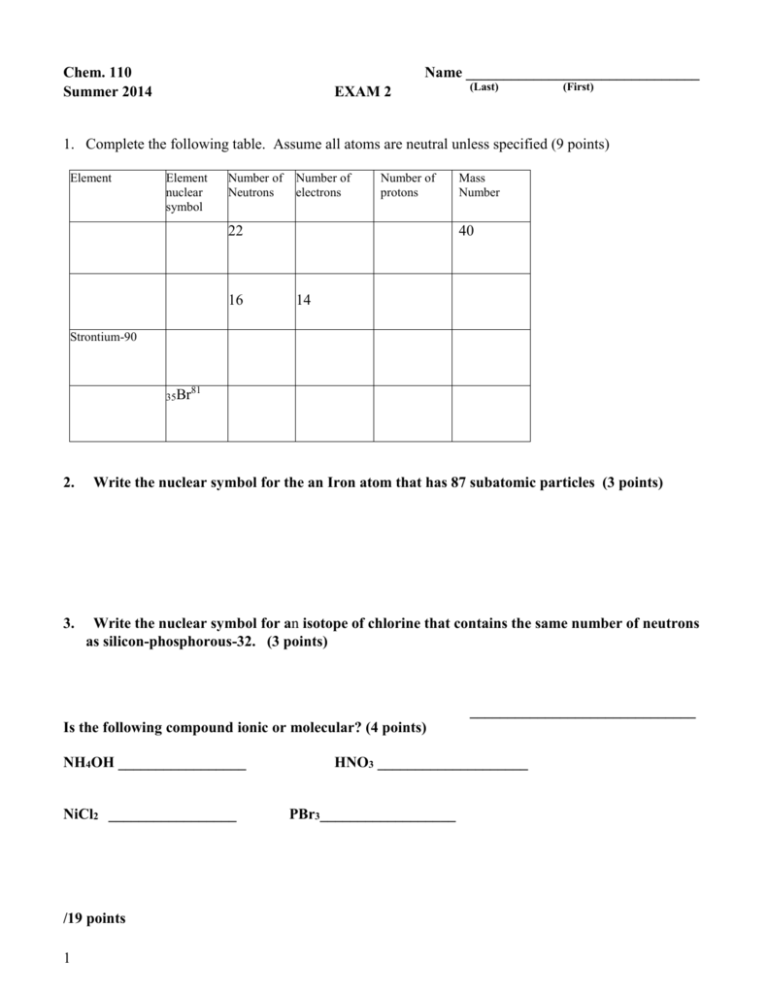
Chem. 110 Summer 2014 Name _______________________________ EXAM 2 (Last) (First) 1. Complete the following table. Assume all atoms are neutral unless specified (9 points) Element Element nuclear symbol Number of Neutrons Number of electrons Number of protons 22 16 Mass Number 40 14 Strontium-90 81 35Br 2. 3. Write the nuclear symbol for the an Iron atom that has 87 subatomic particles (3 points) Write the nuclear symbol for an isotope of chlorine that contains the same number of neutrons as silicon-phosphorous-32. (3 points) ______________________________ Is the following compound ionic or molecular? (4 points) NH4OH _________________ NiCl2 _________________ /19 points 1 HNO3 ____________________ PBr3__________________ 4. Write the formula for each of the following (5 points) a. chloric acid _________________________ b. aluminum dihydrogen phosphate _________________________ c. zinc acetate _________________________ d. silver bisulfite _________________________ e. ferric nitrite _________________________ /10pts. 1. Write the Name for each of the following (5 points) 2 a. (NH4)2C2O4 _________________________ b. S3N2 _________________________ c. CrI3 _________________________ d. BaCrO4 _________________________ e. H2SO4(aq) _________________________ Problems For each of the following problems give the complete setups including all units. Use dimensional analysis when possible. Present your work in a neat and organized manner. If work is not shown, NO CREDIT will be given for your answer. 1. How many atoms of oxygen are there in 2.453 grams of aluminum sulfite? (8 points) Answer: ________________________ 2. What is the total number of atoms in 1.50 X 104 grams of caffeine C8H10N4O2 ? (molar mass C8H10N4O2 = 194 g/mole) (8 points) Answer: ________________________ 3. How many grams of oxygen are in a sample of aspirin (acetylsalicylic acid C9H8O4 )that contains 2.56 grams of hydrogen. (molar mass = 180.16 g/mole)? (8 points) Answer: ________________________ 4. How many grams of nitrogen are in 4.369 x 10 18 molecules of nitroglycerine, C3H5O9N3? (molar mass C3H5O9N3 = 467.3075 g/mole)? (8 points) Answer: ________________________ /32pts. 3 5. A 18.65 gram sample of copper metal is burned in a nitrogen atmosphere to produce 21.39 g of a copper-nitrogen compound. Determine the compound’s empirical formula. (11 points) (This is similar to what you did in the magnesium oxide lab.) Answer: ________________________ 6. What is the mass in grams of one single carbon monoxide molecule? (10 points) Answer:__________________________ /21pts. 4 7. (14 points) A 0.8640 gram sample of the compound responsible for the pungent odor of rancid butter containing only H, C, and O is burned in a combustion analysis apparatus. The mass of CO2 produced is 1.727 g, and the mass of water produced is 0.7068 g. What is the empirical formula of the compound? Answer: ________________________ 8. If a compound with the empirical formula C5H10O2 has a molar mass of 408.5 g/mole, what is the molecular formula of the compound? (6 points) Answer: ________________________ /20pts. 5











