Density, Molecular Weight, Percent Composition, and Empirical
advertisement

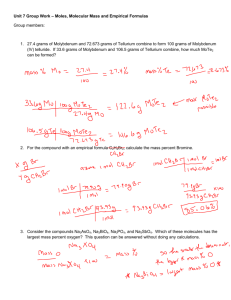
Name ____________________ Period ____ Molecular Weight, Percent Composition, and Empirical/Molecula Formula Review 2014 1. Calculate the molecular weight of C2H6O. 2. Calculate the molecular weight of (NH4)2SO4 3. Calculate the molecular weight of C8H10N4O2 4. Calculate the molecular weight of K4Fe(CN)6 5. Calculate the percent composition of all elements in the following compounds: a. CaC2O4 b. NaC2H3O2 c. Al2(CO3)3 d. Ca(ClO)2 6. Suppose you had 210 grams of Pt(NH3)2Cl2. How many grams of Platinum are there? 7. Suppose you had 15 grams of Co2(CO)8. How many grams of Carbon are there? 8. Suppose you had 215 grams of BaCl2 x 2 H2O. How many grams of water are there? 9. Suppose you had 130 grams of CuSO4 x 5H2O. How many grams of water are there? 10. Suppose you had a compound that was 15.77% Aluminum, 28.11% Sulfur, and 56.12% Oxygen. What is the empirical formula? 11. Suppose you had an organic compound made of 92.25% Carbon, and 7.75% Hydrogen. What is its empirical formula? If the molecule had a mass of 78, what is its molecular formula? 12. Fructose is the sugar found in honey and fruits. It is commonly known as fruit sugar. Its percentage composition is 40.0% Carbon, 6.71% Hydrogen, and the remainder is oxygen. The molar mass of fructose is approximately 180 g/mol. Find the empirical and molecular formulas of the compound. 13. A sample of a pure compound is found to be made up of 1.61 g of phosphorus and 2.98 g of fluorine. Find the empirical formula of the compound. 14. A 145 grams sample of a compound that contains only phosphorus and oxygen was analyzed and found to contain 63.28 g of phosphorus. Calculate the empirical formula. 15. A sample of hydrocarbon undergoes combustion to produce 4.40 g of CO2 and 2.70 g of H2O. What is the empirical formula of this compound? 16. A hydrocarbon undergoes combustion to produce 0.845 g of CO2 and 0.173 g of H2O. What is the empirical formula of this compound? 17. A 0.2500 g sample of a compound known to contain carbon, hydrogen, and oxygen undergoes combustion to produce 0.3664 g of CO2 and 0.1500 g of H2O. What is the empirical formula of this compound?